
The Project Gutenberg EBook of Scientific American Supplement, No. 611, September 17, 1887, by Various This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.net Title: Scientific American Supplement, No. 611, September 17, 1887 Author: Various Release Date: October 26, 2005 [EBook #16948] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN *** Produced by Juliet Sutherland and the Online Distributed Proofreading Team at www.pgdp.net
The motor of MM. Schaltenbrand & Moller is adapted for use for household purposes, where small power is required, as in driving sewing machines.
Fig. 1 shows the motor with all its parts in side elevation, the flywheel and head rest being in section. Fig. 2 is a side view, with the air reservoir and distribution valve in section through the line 1-2. Figs. 3 and 4 represent the same apparatus, but without support, as where it is to be used on the table of a sewing machine, with the crank of the motor directly fastened to the flywheel of the sewing machine. Fig. 5 is a plan or horizontal section at the level of the line 3-4, and Fig. 6 is a section passing through the same line, but only including the cylinder and axis of the distributing valve. Fig. 7 is a horizontal section of the button of the cock through the line 5-6 of Fig. 3. Finally, Fig. 8 shows in detail, plan, and elevation the arrangement of the starting valve.
This little motor does not show any new principle. It uses the old oscillating cylinder, but it embraces in its construction ingenious details which render its application very simple and very easy, especially, as we have already said, to sewing machines.
In the first place, the oscillating bronze cylinder, A, is cast in one piece with the distribution cock, a, Fig. 3, and its seat, b, also of bronze, is adjusted and fastened by means of the screw, b, to the air reservoir, C', cast with its cistern, C, acting as foundation or bed plate for the motor. This cistern is held either on the base of the cast iron bearing frame, D, of the main shaft, d, d, Figs. 1 and 2, or directly on the sewing machine table, Figs. 3 and 4, by means of two pins, e and e', so that it can oscillate about an axis which is perpendicular to the shaft, d, to which is attached the disk, F, carrying the crank.
This arrangement of parts, in combination with the horizontal axis of the distribution valve and with the piston rod, g, considered as a vertical axis of rotation, forms a species of universal joint between the crank pin and the table, so that it can be put in place without adjustment by any workman, who only has to screw up the two screws, h, to fasten to the table the standard, E, and the piece, E', in which are screwed the pivots, e and e', which support the tank, and this all the rest of the motor.
As is seen more clearly in Fig. 2, the water under pressure enters by the pipe, c, to which is attached a small tube of India rubber, and leaves by the pipe, c', and is carried away by another India rubber tube.
The openings of the distribution cock are symmetrically pierced in the seat and plug, which latter is divided internally by a horizontal diaphragm so arranged that at each oscillation communication is established alternately above and below the piston. So that it can be started or stopped quickly, the opening and closing of the throttle valve, i (Fig. 2), is effected by a single pulling movement upon the handle, I, and this draws out the valve horizontally. For this end the lever is pivoted upon the extremity of the valve stem, and ends in a bar engaging with a fork which acts as its fulcrum. This fork is cast in one piece with the plug, J, which closes the opening through which the valve is put in place, as shown in detail in Fig. 8. To prevent the lever from spinning out of the fork when it is pulled or pushed, this lever is prevented from turning by the valve stem, provided for this purpose with a double rib, i' (Figs. 2 and 8), which engages in slots in one piece, j, secured in the interior of the plug, J.
Lest the friction of the conical distribution valve oscillating with the cylinder should occasion a loss of power, care is taken to leave the key free in its seat, b, by not forcing the pivot, k (Figs. 1, 3, and 5), whose position in its seat is regulated by the screw, k'. It follows that a very slight escape of water may be produced, but that does no harm, as it is caught in the reservoir, C, provided with a little pipe, K (Figs. 1 and 3), to carry it away.
To maintain proper relations between the pressure of the water, or the work it is called upon to do, and the motor, the quantity of water introduced into the cylinder at each stroke of the piston is regulated by adjusting the length of stroke by the crank pin. For this end the course of the latter is made variable by means of the piece, f, adjusted by set-screw in the interior of the disk, F (Figs. 3 and 7), and tapped for the reception of a screw terminated by a milled button, f. If this button is turned, it moves the piece, f, and therefore regulates the distance of the crank pin, g', to which the piston rod, g, is attached (Fig. 3) from the center of rotation.
When the motor is arranged as shown in Figs. 1 and 2, or for the transmission of motion by means of a band wheel, p, cast in one with the flywheel, P, the disk which receives the crank pin of variable position is fixed directly upon the axle, d, of the same flywheel carried by the support, D; but when the motor can be applied directly, as is the case for example in the Singer sewing machine, upon the axle of the machine, no support is used, and the arrangement shown in Figs. 3 and 4 is adopted. In this case the disk, F', is cast with three arms which serve, by means of a screw, to fasten it to the flywheel carried by the axle of the sewing machine.
When the motor is used on the upper stories of buildings, the changes of speed incidental to drawing the water from the lower stories from the same pipe can be compensated by the use of an accumulator. This accessory apparatus is composed of a reservoir of a capacity of 10 liters or more, intercalated in the pipe which supplies the motor, so that the water coming from the principal pipe enters the bottom of this reservoir, passing through an India rubber valve opening inward, the supply for the motor coming through a tube always open and placed above this valve. The air trapped in the accumulator is compressed by the water, and when the pressure in the pipe decreases, the valve closes and the compressed air drives the water through the motor with decreasing pressure until normal pressure is re-established in the pipes.—Publication Industrielle.
Some important trials of the new machinery of the screw steamer Ohio, belonging to the International Navigation Company, have recently taken place on the Clyde. The Ohio is an American built steamer measuring 343 ft. by 43 ft. by 34 ft. 6 in., and of 3,325 tons gross. She has been entirely refitted with new engines and boilers by Messrs. James Howden & Co., Glasgow, who also rearranged the bunker, machinery, and hold spaces, so as to give the important advantage of increased cargo accommodation obtainable from the use of their improved machinery, which occupies considerably less space than the engines and boilers of the same power which have been replaced. The new engines are of the triple expansion type, and the boilers, which are designed for supplying steam of 150 lb. pressure, are worked on Howden's system of forced draught, which combines increased power with high economy in fuel. The object of the owners in refitting the Ohio was to test the capability and economy of this system of forced draught on a sufficient scale to guide them in dealing with steamships of the largest class and great power.
In the refit of the Ohio the boilers were designed to work with a very moderate air pressure, this being sufficient for the power required by the contract. The combined power and economy, however, guaranteed by Messrs. Howden & Co. for the use of their system of forced draught was higher than has hitherto been attempted in any steamship, and sufficient, if attained, to prove the large reduction that could safely be made in the number and size of boilers for the use of the system, and the quantity of coal required to produce a given power. The contract for the refit of the steamer required that 2,100 indicated horse power (which was the maximum power of the engines removed) should be maintained during the trial on a consumption of 1.25 lb. of coal per indicated horse power per hour. Originally the boilers of the Ohio, from which this power was produced, were three in number, double ended, 12 ft. 6 in. in diameter by 17 ft. 6 in. in length, having each six furnaces 3 ft. in diameter, or eighteen furnaces in all, with an aggregate fire grate area of 300 square feet. The new boilers, fitted with the forced draught, are likewise three in number, but single ended, 13 ft. in diameter by 11 ft. 2 in. in length, having each three furnaces 3 ft. 3 in. in diameter, or nine furnaces in all, with an aggregate fire grate area of 112 square feet. Air for combustion is supplied to the boilers by one of Messrs. W.H. Allen & Co.'s fans, 5 ft. 6 in. in diameter, driven direct by an engine having a cylinder 7 in. in diameter with stroke of 4 in. The boilers removed had two stoke holds across the ship, one fore and one aft of the boilers, while the new boilers have only one stoke hold on the after side. The engines removed have cylinders 57 in. and 90 in. in diameter by 48 in. stroke, while the new engines have three cylinders 31 in., 46 in., and 72 in. in diameter respectively, with piston stroke of 51 in.
During the trials the coals were weighed out under the supervision of the officers of the company, who also took the record of speed and other data. After running down Channel for a considerable time, the trial on the coals weighed out began, and lasted 4 hours 10 minutes, during which time 10,885 lb. of Welsh coal were burned, the trial ending with the same revolutions of engines and the same pressure in boilers with which it began. The mean indicated horse power, calculated from the mean of seven sets of indicator cards, taken during the trial, and the mean revolutions per minute, found by dividing the total revolutions recorded on the engine counter by the minutes in the period of the trial, amounted to 2,124, thus making the consumption 1.23 lb. per indicated horse power per hour, and the power per square foot of fire grate almost exactly 19 indicated horse power. While testing the indicated horse power and consumption of coal, the steamer ran to and fro between the Cloch and Cumbrae lights, and also made several runs on the measured mile at Skelmorlie, from which the mean speed of the vessel was found to be 14.12 knots per hour. The remarkably high results obtained were most satisfactory to the representatives of the owners, and a large party of experts on board congratulated Mr. Howden on the successful fulfillment of the onerous guarantees undertaken.—Engineering.
The works illustrated by the engravings are now being constructed under a concession from the imperial government of Brazil. The province of Ceara has an area of about 50,000 square miles, and is one of the richest in Brazil. Its produce comprises sugar, coffee, cocoa, cotton, tobacco, spices, fruit, cabinet and dye woods, India rubber, etc. Its population at the last census, taken in 1877, amounted to 952,624 inhabitants, that of the capital, the city and port of Ceara, being about 40,000. Although Ceara is the principal seaport at which lines of English, French, American, Brazilian, and other steamers regularly call, prior to the commencement of the harbor improvements it was almost an open roadstead, passengers and goods having to be conveyed by lighters and boats between vessels and the shore. The official statistics of the trade and shipping of the port show that an income of £35,750 per annum will be collected by the Ceara harbor corporation from the dues which they are authorized by their concession to charge on all imports and exports and on the vessels using the port and from the rent of the bonded warehouses.
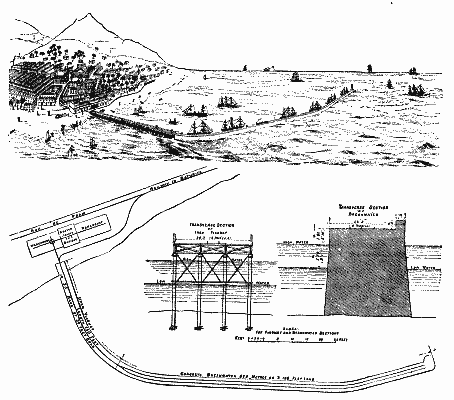
The drawings given here show the nature of the works, which are of a simple character. The depth of water along the principal quay, which is being constructed of solid concrete, and is connected with the shore by an iron and steel viaduct over 750 ft. in length—which is already completed—will be 19 ft. at low water and 25 ft. at high. This quay and breakwater is shown in perspective, in plan, and in section, and is of a very heavy section, as will be gathered by the scale given immediately below it. Meanwhile the landing of cargo is temporarily carried on at the end of the viaduct, which at high tide has a depth of about 20 ft. of water. The custom house and bonded warehouses are being built of the fine granite obtained at the Monguba quarries, which adjoin the Baturite railway, about sixteen miles from the port. A new incline has also been constructed from the rail way down to the port. The line has been laid along the viaduct, and will be extended over the quays as soon as they are completed. The concrete, of which a large quantity is being used, is mixed by Carey & Latham's patent mixers, and the contractors have supplied the very large and complete plant for carrying out the operations.
The engineer to the corporation is Mr. R.E. Wilson, M. Inst. C.E., Westminster, and his resident at Ceara is Mr. R.T.H. Saunders, M. Inst. C.E. The contractors for the work are Messrs. Punchard, McTaggart & Co., their representative at Ceara being Mr. George Wilson, M. Inst. C.E.—The Engineer.
Why should we prefer electricity as the propelling agent of our street cars over all other known methods? I answer, without hesitation, because it is the best, and being the best is the cheapest. Briefly I will present the grounds upon which I take my stand.
To-day the only methods for tramway service are three in number: Horses, with a history of fifty years and over; cables, with a history of fifteen years; and electricity, with a history of two years. I give the latter two years on the basis of the oldest electric street railroad in existence to-day, and that is the Baltimore railroad, equipped with the Daft system.
The main points for consideration common to each are six in number:
Each of these requires a paper by itself, but in as concise a way as possible, presenting only the salient reasons and figures, I shall endeavor to embody it in one.
1st. Obtaining of franchise.
I assume the municipal officers and the promoters honest men.
It is the universal settled conviction that a street car propelled with certainty and promptness by mechanical means is infinitely to be preferred to horses. Hence, if this guarantee can be given, there need be no fear from the other side of the house. Years of experience prove that this guarantee can be given.
The mechanical methods are electricity and the cable. To suit local conditions the former has three general applications—overhead, underground, and accumulator systems; while the latter has but one, the underground. Hence, the former, electricity, has three chances to the latter's one to meet the whims, opinions, or decisions of municipal authorities. Other advantages accruing from mechanical methods are cleaner streets, absence of noise, quick time, no blockades, no stables accumulating filth and breeding pestilence, and lastly the great moral sympathetic feeling for man's most faithful and valuable servant, the horse. These all are directly in favor of obtaining the right franchise.
The three general ways of obtaining the same are a definite payment of cash to the authorities, a guarantee of an annual payment of a certain per cent. of the earnings, and lastly a combination of the two. For the city or town the latter way is the safest, and the best, all things considered. As electricity is mechanical, and as it can be shown that it is the cheapest to construct and most economical, and has three chances to operate, it stands by far the most likely to obtain the franchise.
2d. Construction of buildings.
The governing factors under this head are the local land valuation and tax. The system necessitating a spread eagle policy on the land question will cost. What could be a more perfect illustration than the horse railroad system? The motive power of the New York Central Railroad between New York and Albany could be comfortably stowed in the barns of some of the New York City street railways. What a contrast! The real estate, buildings, and fixtures of the Third Ave. line are valued at $1,524,000, and what buildings! Cattle sheds in the metropolis of America. Surely they did not cost a tithe of this great sum. What did? The land, a whole block and more. Henry George advocates might find food for thought here. All this is true of the other lines in every city in the Union. Enormous expenditures for land. A good one half of their capital sunk in purchasing the necessary room. Go where you will, a good fifty per cent. of the capital is used for land for their stables. This obviously does not include equipment.
How is it with mechanical systems? The land is one of the minor considerations, the last thing considered. Let us look at some figures. From careful examination of many engine plants, considering the ratio between a certain number of horses with their necessary adjuncts and a steam plant of numerically equal power, I find it stands as 1 to 30. That is, a steam plant complete of 30 horse power capacity would need only one thirtieth the floor space of thirty horses. With larger powers this ratio is still greater, and from one estimate I found that it stood as 1 to 108, i.e., for horses I should have to have 108 times more floor space than for an equal number of mechanical horse power. It must be remembered also that the mechanical horse power is 50 per cent. greater than the best animal horsepower.
From one maker, taking the engine alone, I found that a rated 100 horse power engine, guaranteed in every particular, would have ample room in the stall for one horse in the average stable. Another instance showed that I could get a steam plant complete, engine, boiler, etc., of 50 horse power, in a space 5 by 6 feet, which is smaller than the average stall. Here is shown the enormous saving in land purchase.
For car room a building several stories high would answer perfectly, since quick-hoisting elevators could be put in and the tracks on each floor have wire connections with the dynamos, so that the cars could be run across the floor to where you please, facilitating storage and dispensing with handling. This would not be possible with the cable.
Comparing electricity and cable on this point, all things favor the former clearly and beyond all question. Furthermore, if locality so favored, the subject of land purchase for electricity could be tabooed entirely, since distance can be so readily overcome. Way out in the suburbs or back in the country by the side of some waterfall, your station might be, while the current is sent to the great city over heavy conductors. Here land rent or tax would be at the minimum. With horses or cable plainly proximity must be had. It is estimated that the land occupied by the Madison Avenue line of New York City is worth the cost of 40 miles of ordinary double track.
3d. Equipment at station and rolling stock.
The rolling stock would be in each case approximately the same. Consisting of cars of equal seating capacity, the difference of cost would be the necessary attachments for the mechanical systems.
I believe, however, that the mechanical system is bound to work material changes in car construction, in fact it is almost imperative. In all probability a car with 15 to 20 per cent. greater seating capacity than the horse car can be constructed on a different plan for the price given for the electric car. This price, it must be noted, is the one for attachment of motor to the present horse car. The horse cars produced to-day are most carefully planned, thoroughly built, and admirably adapted to their service, but the inexorable law of progress decrees their extinction, for something better.
Motive power. To represent clearly the costs, etc., of the three systems under this head, let us assume a road. Take, if you please, a double line 6 miles long, and operating 24 cars with speed of 6 miles an hour, and running 20 hours out of 24. This would call for 48 horses on the track and 192 horses in the stables, or a total of 240 horses; at $160, counting harness, etc., this would cost $38,400.
With electricity we will proceed as follows: The weight of car with 30 passengers and motor attachments would be about 9,000 lb. It is easily calculated that to propel the same at the specified rate on a level would take about 1.75 horse power, a total of 42 horse power. To make allowances for grades we can calculate that, if the entire road was one gradient of three per cent., each car would take about 6.4 horse power, or since only 12 are going up, a total of 76.8 horse power. It will be fair now to take the average of these two, or 59.4 horse power for an average road. Allowing 35 per cent. loss from engine to work done in actually propelling car, we would have to have 91.3 horse power. Allowing a good safety factor, it would be well to put in a 150 horse power plant. This would cost complete $7,000; necessary dynamos, $3,500. Among these figures should be counted cost of conductor of sufficient size to allow of but three per cent. in energy to overcome its resistance. This I have calculated using a potential of 600 volts; and find that the total cost of six miles copper conductor is $16,000 with above conditions. The total cost is now seen to be $26,500.
As to cables, since the recovery of energy available for tractive purposes is but 35 percent., then the engine of 169 horse power represents what must be had. Allowing a generous factor of safety, let us say that a 250 is all sufficient. This would cost complete and erected about $12,000. The cable would cost $15.000, and gears, etc., $8,000, making a total of $35,000.
The ratio of the three systems stands: Electricity, 1; cable, 1.09; horse, 1.45.
4th. Construction of tramway.
Figures upon this point must necessarily be either averages or approximations. The nature of the locality socially, naturally, and we grieve to say it, politically, has a strong influence upon its construction. Estimating on single track only, a horse road would cost as an average $9,000 per mile. With electricity we have several methods we can avail ourselves of: Surface, costing about $10,000; overhead double conductor, $15.696; underground, $23,500.
With cable but one method, the underground, is possible. This cost is variously estimated at from $30,000 to $110,000 per mile; however, the latter figure is excessive. A fair average would be $35,000.
The ratio of constructions could be fairly placed as follows, putting electricity as 1, by taking the average of the three methods at $16,732: Horse road, 0.53; cable, 2.09.
Unquestionably a great majority of roads of the past have not been constructions of engineering, and of all places requiring care, skill, and engineering, the street roads are the places.
5th. Cost of operation.
A fair figure for cost of one horse for one year is $220.
For electricity, allowing 35 per cent. loss in transmission, etc., 1.54 horsepower would be the work done by engine to get 1 horse power on the track. There are to-day plenty of steam plants producing 1 horse power for work at from $30 to $50 per annum. Take the average, $40. With electricity then $65 would well represent the price of producing 1.54 horsepower.
With cable these figures would hold true, but more work is required. A greater loss is entailed. Since but 32 per cent. is recovered, the figure for 1 horse power on the track would be 2.86 horse power. At the above rates this would be $110 per horse power per year.
Our ratio here is: Electricity, 1; cables, 1.71; horses, 3.38.
This is by no means the whole of the story, for just here must we compute the depreciation and hence repairs due to time. Let us take the road figured on heretofore, and make three tables.
In the following I have under each system taken the estimated costs, allowed a fair per cent. for depreciation, summed up and obtained the ratios.
Any figure then like interest, etc., which would not affect ratios, I have omitted.
| ELECTRICITY. | |
| Conductors, 1 per cent. | $160.00 |
| Engine and dynamos, 5 per cent. | 525.00 |
| Cars, 10 per cent. | 5,280.00 |
| Roadway, 10 per cent. | 2,007.00 |
| Total. | $7,972.00 |
| HORSES. | |
| Horses and appurtenances, 20 per cent. | $7,780.00 |
| Cars, 10 per cent. | 2,880.00 |
| Roadway, etc., 10 per cent. | 3,500.00 |
| Total. | $11,740.00 |
| CABLES. | |
| Cable, 50 per cent. | $7,500.00 |
| Engine and boiler, etc., 5 per cent. | 1,000.00 |
| Cars, 10 per cent. | 4,320.00 |
| Roadway, 10 per cent. | 3,500.00 |
| Total. | $16,320.00 |
These totals put in ratio are as follows: Electricity, 1; cable, 2.04; and horses, 1.47.
Placing all the ratios obtained in a table, we have the following:
| Electricity. | Horses. | Cables. | |
| Depreciation. | 1 | 1.47 | 2.04 |
| Operating expenses. | 1 | 3.38 | 1.71 |
| Construction of tramway. | 1 | 0.53 | 2.09 |
| Motors, cars, etc. | 1 | 1.63 | 1.21 |
| Cars. | 1 | 0.54 | 0.81 |
| Totals. | 5 | 7.55 | 7.86 |
| Average. | 1 | 1.51 | 1.57 |
Now this table must stand by itself for what it represents, and no more. It will be noted that I have not introduced the subject of men. This would unquestionably show favorably for both electricity and cable. Again, note, please, that this table does not represent your profits exactly as per ratios. I have to get them operated the same number of cars and under the same headway. Now with either electricity or cable a higher rate of speed can be maintained with but a very small proportionate increase of cost. This means quicker time, more trips, and greater receipts.
Evidently, as a financial investment, even if cost of maintenance and operating is greater, the cable is to be preferred to horses.
How is it with electricity? The ratios of expenses, etc., stand for themselves, the law of speed is far simpler than with cable, bringing even greater receipts, and again in practice the saving of coal in proportion to work done on track day or night is immensely more economical than with the cable. This point will be touched upon later.
6th. Individual characteristics and advantages.
Under this head a few of the salient features of each system will be mentioned. As the possibilities and limitations of the horse railroad system are, however, so well known, it is needless to go over them. I therefore will confine myself to the electric and cable systems.
With electricity single track lines, crooked streets, all descriptions of turnouts, crossings, branches, etc., are as easy to construct and operate as with horses. With the cable system they are either impossible or enormously expensive.
With electricity the line is not a unit, so that the complete stoppage of the whole line is absolutely impossible. With cable it is a unit and it is possible.
With electricity the life of the conductor is infinite; with cable, two years.
With electricity, and the improvements now being made in traction wheels, etc., the heaviest grades are as easily surmounted as with the cable; although it is true that for grades exceptional in character, such as 20 per cent. grades or over, I should be willing to give the contract to cable.
With electricity any speed can be attained by the individual cars. They are absolutely independent. Lost time can be made up, etc. With cable the cars are dependent upon speed of cable. Lost time cannot be made up except on down grades.
With electricity work done by engine is synchronous with work done on the track at any time of the day or night, with the loss of 35 per cent. due to the conversions in each case. In other words, for every horse power of useful work done on track the engine does 1.54 horse power. This ratio is constant. It makes no difference whether 1 or 100 horse power of work is necessary on the track, the engine has but to do 35 per cent. in excess.
With cable, if 1 horse power of work is all that is required on the track, the engine may be doing 25 horse power to get that amount there through the gears and cable. With heavier loads this is somewhat diminished, but about the very best figure that can be put forth is but 35 per cent. recovery, with 65 per cent. loss—the exact converse of electricity under heavy loads.—Street Railway Journal.

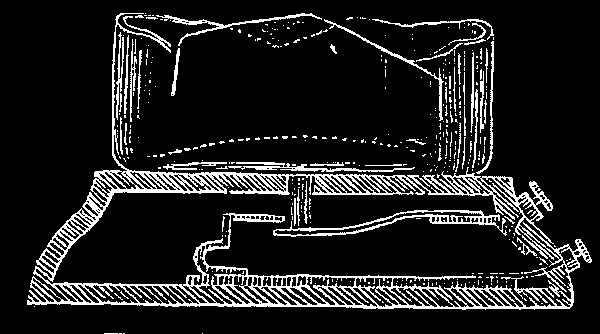
To avoid the errors which sometimes occur in a pharmacy or in a laboratory, where one bottle is taken for another, especially in the case of those containing highly poisonous or dangerous substances, a simple arrangement, shown in the cuts, has been proposed. The apparatus, in principle, is a species of electrical alarm, in circuit with an ordinary house telegraph line. It consists essentially, as shown in Fig. 1, of a battery, bell, and pedestal, provided with an electric contact on which the flask rests. Fig. 2 shows this contact or break piece. On a series of pedestals thus arranged and intercalated in the same circuit the flasks containing poisonous or dangerous substances, whose inadvertent handling might cause trouble, are placed. In removing one of these flasks the circuit is closed, and the electric bell notifies the pharmacist of the danger attendant on the use of the substances contained in the flask referred to, thus guarding against the errors due to carelessness, and quite too frequent, especially in pharmacies.—Chronica Cientifica.
The following apparatus, constructed after the designs of Dr. Loeb, assistant in the Physiological Institute at Wurzburg, is for the purpose of measuring the reaction period of hearing, that is, the period which elapses between the time when a sound wave affects the auditory nerve and is thence transferred to the brain, then affecting the consciousness, and the moment when the motor nerves can be thrown into action by the will. It is, therefore, necessary to fix both instants—when the sound is produced and when the observer has, from its warning, received the impulse so as to press down a key. The great advantage of this instrument over others adapted for the same end consists in this, that the determination in its essentials is effected entirely by mechanism, and, therefore, the graphic results attained by it are free from all sources of error, which errors other methods always introduce to a greater or less extent. Thus its results are quite unexceptionable.
The apparatus shown in the cut rests on three feet, two of them consisting of strong screws, so that by aid of the circular level, l, on the base plate, it can be adjusted perfectly level. On a little shelf attached to a square rod, seen on the left of the instrument, rising from the base plate, and near its top, is a horizontal tube, through which, by a bulb not shown in the cut, a blast of air can be blown. In front of the other opening of the tube is a horizontal fork of ebonite, whose arms carry on the side opposite the tube a metallic ball. Through the arms of the fork pass the wires of the circuit of an electric battery. These terminate in two rounded ends, which, when the arms approach each other, are touched by the metallic ball, so that the latter also closes the metallic circuit. By the blast of air a wooden wedge contained in the tube is driven between the arms of the fork, the ball falls from them, and the electric stream is cut off. The ball drops upon the inclined metallic plate, p, bounces off it, and is received in a little sack, S. When the observer hears the ball strike the plate, he presses on the key, t, and the interval between the two instants, namely, the falling of the ball upon the plate and the pressing of the key, t, is what is to be mechanically fixed and measured.
The electric current, which is closed by the ball as long as it lies on the jaws of the fork, flows around the arms of the electro-magnet, m, which continually attracts an armature fastened to a lever arm, and coming over the poles of the magnet. If the circuit is broken by the fall of the ball, the armature at once rises upward. By this a spring contained in the tube, g, and hitherto kept compressed, is released, which gives a shock to the right angled ½frame, a a, containing a blackened or smoked plate of glass, so that, following the wire, b, acting as a guide, the plate flies from left to right of the apparatus. To prevent the plate from recoiling, a catch, d, is fastened to the side bar, c. Furthermore, lest the friction of the wire, b, in the guiding apertures of the frame should impair its velocity as it moves from left to right, it is connected with a weight pan by a cord passing over the pulley, g, which is so loaded that by the added velocity with which it strives to fall, the retardation already alluded to is overcome, so that the frame moves from left to right with even speed.
In front of the frame, a a, is the tuning fork, f, which as estimated makes 184 vibrations in a second. By the stylus, y, on the upper limb of the fork these oscillations are marked upon the sliding plate of glass as a wave line. Lest, after the first impulses of the fork have been registered, they should soon die away, in front of it is an electro-magnet, H, whose pole-faces near the arms of the tuning fork pass over them. The latter, to be more strongly affected by the magnet, are provided with faces of soft iron. To the lower face of the lower arm of the fork a small sharp stylus is fastened, which, with each beat of the fork, comes into contact with the mercury in the little cup, n, or a spring used instead of it. This closes an electric circuit, which passes around the magnet, thence going through the tuning fork by the binding screw, k, and thence by connections not shown in the cut back to the battery. In consequence of the magnetism thus excited, the arms of the tuning fork are attracted by the poles of the magnet, and forced to beat with increased amplitude. In a short time a constant amplitude of oscillation is reached, when the magnetic impulses are of equal influence with the atmospheric resistance and the internal force of the tuning fork restraining its movements.
Finally, the stylus, s, which touches the glass plate directly above y, is for registering the moments when by the falling ball the sound is produced and when the observer presses the key. This is brought about by the rod, i, to which s is firmly screwed, being jerked upward a short distance at each of these instants, so that the horizontal lines which the stylus, s, marks upon the screen passing in front of it are broken at both places.
The mechanism which jerks the rod, i, upward is thus arranged: The inclined plate, p, on which the ball drops, is carried by the upper horizontal arm of an angular lever turning on the axis, x, and counterpoised by the balancing weight, x'. By the falling ball this arm is pressed downward, and the lower horizontal arm, w, of the lever is also moved. On a second horizontal axis the lever, v, partly concealed, moves, restricted as to its length of swing by the screws, n. As long as the concealed arm is not moved, v is lightly pressed by the small spring, e, against w. The projection, z, at the upper end of v holds the rod, i, which the strong spring, h, is continually pressing upward. When the ball falls upon the plate, p, the arm, w, presses against the lower end of v, the projection, z, sets free the rod, and it springs upward. This movement is soon arrested, as the projection, z', engages with a stud situated on the right side of the rod, i. This projection is situated on the vertical arm of an angular lever whose other arm is the key, t. When the observer presses the key, the rod, i, again is jerked upward by the spring, h. The screw, o, tapped into the rod, i, prevents the rod going higher than necessary, by striking a plate, which also serves as guide for i.
To determine the interval between the falling of the ball and pressing of the key, one has finally to count the waves inscribed by the tuning fork, which come under the portion of the line inscribed by s, which is bounded by the two breaks produced by the successive movements of the rod.
To make the glass plate carried by the frame available for more observations, which plate can be used as a photographic negative, the frame, T, is adjustable up and down upon the pillars, N. This frame carries the tuning fork, mercury cup, n, and the electro-magnet, M. The spring, s, can also be moved up and down along the rod, i.—H. Heele in Zeitschrift fur Instrumentenkunde.
The accompanying engravings represent a new disinfecting apparatus invented by Mr. W.E. Thursfield, M. Inst. C.E., of Victorgasse, Vienna. The principle on which its action is based is that the complete destruction of all germs in wearing apparel and bedding, without any material injury whatever to the latter, is only to be obtained by subjecting the articles infected, for a period proportionate to their structural resistance, to a moist heat of at least 212 deg. Fah. Recent experiences in Berlin have shown that, for security's sake, a temperature of 220 deg. is better. To insure the thorough penetration of this temperature in every fiber, a heat of from 260 deg. to 270 deg. must be maintained in the disinfecting chamber itself. To obtain this by means of ordinary or superheated steam involves the employment of boilers working under a pressure of 2½ to 3 atmospheres, of disinfecting chambers capable of resisting an equal tension, and of skilled labor in attending to the same; in other words, a large initial outlay and correspondingly heavy working expenses in fuel and wages.
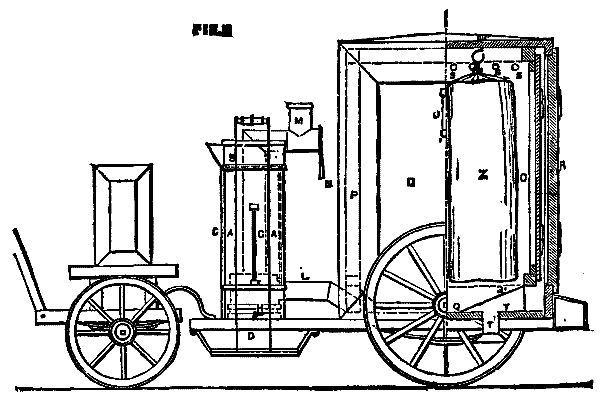
The disinfecting apparatus, illustrated in a portable and stationary form, of the dimensions adopted by the sanitary authorities of Vienna, Budapest, Prague, Lemberg, Teplitz, etc., and by the Imperial and Royal Theresianum Institute, and sanctioned for use in barracks, military hospitals, etc., by the Austrian Ministry of War, and for ambulance hospitals by the Red Cross, acts by means of a mixture of steam and hot air in such proportion that the steam, after expending its mechanical energy in inducting the hot air into the disinfecting chamber, is, by contact with the clothes or bedding of a lower temperature, not only condensed, but by condensation completely neutralizes the risk of injury through any chance excess of hot air. The boiler being practically open is inexplosive, and requires neither safety valves nor skilled attendance.
The heat generated in the furnace is utilized to the utmost, and the escaping vapors form a steam jacket in the double casing of the disinfecting chamber. The method of manipulation reduces the danger of contagion to a minimum, as the clothes or bedding are placed in specially constructed sacks in the sick chamber itself, and, after being tightly closed, the sacks are removed and hung in the disinfector. The stationary apparatus, which is constructed to disinfect four complete suits of clothes, including underlinen, or one complete set of bedding, including mattress, is specially adapted for hospitals, barracks, jails, etc. Its dimensions can easily be increased, but the size shown has proved itself, from an economical point of view, the best, as, where the quantity of articles to be disinfected varies, several apparatus can be erected at a less cost than one large one, and one or more be heated as the quantity of infected articles be small or large. In the accompanying drawing A is the boiler, which is filled by pouring water into the reservoir, B, until the same, entering the boiler at its lowest part through the tube, C, rises to the desired height in the water gauge, G. C acts also in the place of a safety valve. D is the fire space, E a movable grate, and F the coal hopper. The fuel consists of charcoal or coke. The boiler is emptied by the cock, H. I is a steam pipe connecting the steam space with the hot air tube, L¹. K is an auxiliary pipe to admit the steam into the chimney during stoppage for emptying and recharging the disinfecting chamber in continuous working. The admission of air is regulated by the handle, L, and the draught in the chimney, M, by the handle, N. O is the disinfecting chamber inclosed by the space, P, which acts at the same time as a steam jacket and as a channel for the downward passage of the vapors escaping from the chamber through the outlets, S. The lower portion of the disinfecting chamber, Q, is funnel-shaped for the better mixture and distribution of the steam and hot air, and to collect any condensation water. Q¹ is a sieve to catch any fallen article. The vertical tubes, S, which serve at the same time to strengthen the chamber, connect the lower portion of the steam jacket, P, with the circular channel, T, which is again connected with the chimney, M, by the tube, T'. The disinfection chamber is hermetically closed by the double cover, R, to the lower plate of which hooks for hanging the sacks are fastened. The cover fits in a sand bath, and is raised and lowered by means of the pulley chain, W, and the swinging crane, X. U is a thermometer indicating the temperature of the steam and hot air in the disinfecting chamber, V a cock for drawing off any condensation water, Y a battery connected with an electrical thermometer to be placed in the clothes or bedding, and Z the sacks in which the infected articles are hung.
The portable apparatus, as shown, for heating with gas, or even spirits of wine, can also be heated with a similar steam and hot air apparatus as the stationary disinfector. In country towns or villages, or even in cities, whose architectural arrangements permit, the portable disinfector can easily be drawn by one man into the courtyard or garden of any house, and the process of disinfection conducted on the spot. Its usefulness in campaigns for ambulance hospitals is self-evident. The letters denoting the several parts are the same as in the stationary apparatus. The portable disinfector is constructed to disinfect two complete suits of clothes or one mattress. The extremely favorable results are shown in the accompanying table of trials.—The Engineer.
TABLE OF RESULTS WITH WM. E. THURSFIELD'S STEAM AND HOT AIR DISINFECTORS.
| Series of Trials. | I. | II. | III. | IV. | V. | VI. | VII. | VIII. | IX. | X. | XI. | XII. | XIII. | XIV. | XV. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Portable Apparatus. | Stationary Apparatus. | ||||||||||||||
| Contents of boiler, in gallons | 3.85 | 4.18 | — | 4.18 | 4.18 | 4.18 | 5.7 | 5.7 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Water added during the process | — | 1.54 | — | — | — | — | 1.4 | 0.6 | 4.3 | — | — | 7.4 | 1.4 | — | — |
| Temperature of water degs. Fah. | — | — | — | 72 | 57 | 54 | 43 | 132 | 54 | 46 | 176 | 43 | 43 | 43 | 104 |
| Firing commenced with spirits of wine at hours min. | — | 2.12 | 9.10 | 4.30 | — | 10.0 | — | — | — | — | — | — | — | — | — |
| Firing commenced with gas at hours min. | 1.30 | — | — | — | 3.0 | — | — | — | — | — | — | — | — | — | — |
| Firing commenced with coke at hours min. | — | — | — | — | — | — | — | 1.10 | — | 8.15 | 1.13 | 1.43 | 2.54 | — | — |
| Firing commenced with charcoal at hours min. | — | — | — | — | — | — | 10.12 | — | 2.15 | — | — | — | — | 8.43 | 10.16 |
| Steam generated at hours min. | — | 2.34 | 9.28 | 4.41 | 3.15 | 10.18 | 10.35 | 1.34 | 2.38 | 8.53 | 1.20 | 2.3 | 3.19 | 9.3 | 10.23 |
| 212 deg. in chamber registered by external thermometer at hours min. | 2.30 | 2.40 | 9.34 | — | — | — | 10.50 | 1.52 | 2.45 | 9.3 | 1.28 | 2.18 | 3.37 | 9.12 | 10.31 |
| 212 deg. in clothes registered by electrical thermometer at hours min. | — | — | — | 5.25 | 4.18 | 12.12 | — | — | — | — | 1.55 | — | — | — | — |
| 221 deg. in clothes registered by electrical thermometer at hours min. | — | — | — | — | — | — | 11.51 | 2.34 | — | — | — | 3.50 | 4.26 | 10.4 | 12.03 |
| Highest temperature in chamber registered by external thermometer deg. | — | 270 | 250 | — | 324 | 255 | 302 | 275 | 293 | 320 | 284 | 284 | 302 | 284 | 275 |
| Mean temperature in chamber registered by external thermometer deg. | 241 | 257 | 239 | 266 | — | 253 | 266 | 266 | 284 | 284 | 266 | 266 | 284 | 266 | 266 |
| Trial closed at hours, min. | 4.45 | 4.10 | 11.4 | 5.45 | 4.30 | 12.30 | 11.51 | 2.35 | 4.30 | 11.0 | 2.10 | 3.50 | 4.35 | 10.10 | 12.03 |
| Max. therm. registered in mattress deg. | 262 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Max. therm. registered in overcoat deg. | — | 239 | 226 | — | — | — | 223 | 223 | 253 | 244 | 226 | — | — | — | 223 |
| Max. therm. registered in winter coat deg. | — | — | — | 232 | 223 | 214 | — | — | — | — | — | 230 | 232 | 223 | — |
| Max. therm. regis'd in winter trousers deg. | — | 243 | 239 | — | — | — | — | — | 262 | — | 253 | — | — | — | — |
| Max. therm. regis'd in summer trousers deg. | — | 246 | 252 | — | — | — | — | — | 280 | — | 264 | — | — | — | — |
| Time required to generate steam min. | — | 22 | 18 | 11 | 15 | 18 | 23 | 24 | 23 | 38 | 7 | 20 | 25 | 20 | 7 |
| Time required to generate 212 deg. in chamber min. | 60 | 28 | 24 | — | — | — | 38 | 42 | 30 | 48 | 15 | 35 | 43 | 29 | 15 |
| Time required to generate 212 deg. in clothes min. | — | — | — | 55 | 78 | 132 | — | — | — | — | 42 | — | — | — | — |
| Time required to generate 221 deg. in clothes min. | — | — | — | — | — | — | 99 | 85 | — | — | — | 127 | 92 | 81 | 107 |
| Total duration of process min. | 135 | 118 | 114 | 75 | 90 | 150 | 99 | 85 | 135 | 105 | 57 | 127 | 101 | 87 | 107 |
| Water evaporated, in gallons | — | — | — | 1.65 | 1.90 | 2.75 | 4.3 | 3.3 | 6.93 | — | — | 9.24 | — | 3.63 | 4.84 |
| Consumption of spirits of wine pints | — | — | — | 3.0 | — | 9.6 | — | — | — | — | — | — | — | — | — |
| Consumption of gas, in cubic feet | — | — | — | — | 70 | — | — | — | — | — | — | — | — | — | — |
| Consumption of cokes, in cbs | — | — | — | — | — | — | — | 6 | — | — | 8.8 | 16.5 | — | — | — |
| Consumption of charcoal, in cbs | — | — | — | — | — | — | 8.8 | — | — | — | — | — | — | 14.3 | 13.8 |
N.B.—In every case, even in the trials V. and X., in which the temperature in the disinfecting chamber rose above 320 deg. Fah., the clothes, owing to the complete saturation of the hot air with live steam, remained absolutely unimpaired.
The column "water evaporated" shows the quantity of live steam passing through the disinfecting chamber averages 13 cubic feet per minute with gas or spirits, and 22 cubic feet with charcoal or coke in the portable and 33 cubic feet in the stationary apparatus. Trials VI., VII., and VIII. took place in open air.
According to trial XII., from 28 to 30 complete suits of clothes can be disinfected at an expenditure of about 75 cbs. of coke per diem.
This arrangement consists in a cylindrical metal or horn mounted lens two to four centimeters long, and magnifying two or three times, and two or three centimeters in diameter, whose side is provided with a contrivance for holding after it has been pushed into place a copying needle, a protractor, etc.
While hitherto the architect in using millimeter paper must hold separately in his hands a magnifying glass and needle, while the engraver holds the engraving tool inclined in one hand and the magnifying glass in the other, or must work under a large lens standing on three feet, it is now possible by a firm connection between the lens and needle or other instrument to draw directly with one hand and under the lens. In the accompanying cut one of these lenses is shown in section, A, in which the glass is set obliquely, in whose focus the needle, a, is held and the field of view is enlarged. A longer description is unnecessary, as the illustration gives the best explanation. It need only be remarked that the stud, s, projecting a little near the glass, is for the purpose of preventing the instrument from leaving the position coinciding with the plane of the drawing. For architects and engineers is provided a small compass, b, of about 2 cm. diameter, for laying off parallel widths, for making smaller scales and the like. In these cases it is substituted for the needle. In like manner for calculating cross profiles by graphical methods, for reading parallel divisions, for estimating areas, or revising maps, a finely divided prismatic ivory rule, c, can be placed under the glass, B, and will do good service. In this case the plane of the lens must be perpendicular to the axis of the tube.
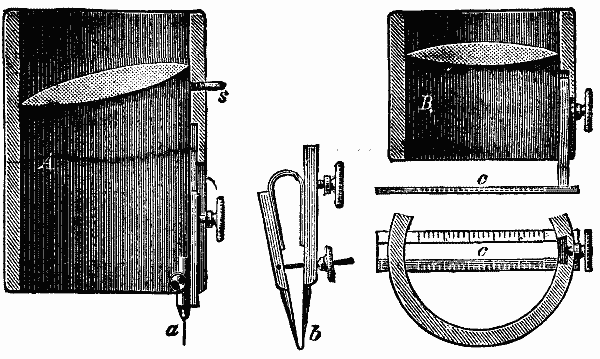
For draughtsmen a parallel drawing pen, something like b, is used, which gives several lines at once, perfectly parallel and close together; or a drawing pen with which the smallest signatures, such as boundary stones and figures, can be made neatly and exactly, which is secured like the needle, a, and for which the cylinder serves also as pen holder, offers a great advance.
Thus a whole series of instruments can be used with the lens. For instance, a naturalist can use with it a knife or other instrument. To avoid injury from the instruments, one should, in laying down the cylinder, place it on its side. It is also recommended that on the outer tube of the frame, which is appropriately lacquered of black color, white arrows should be placed in the direction of the points of the instrument, so that the eyes shall be protected from injury in handling the instrument, as by the points being stuck into the pupil, owing to lifting the instrument in an inverted position.—Zeitschrift fur Instrumentenkunde.
That style of machine for moulding candles in which the candles are forced out at the top by means of a piston is the one most employed, and it is an apparatus of this kind that we illustrate herewith. In its construction, this apparatus presents some important improvements in detail which it is of interest to set forth. The improvements made by the Messrs. Barlow have been studied with a view of manufacturing candles with conical ends, adapted to all chandeliers, without interfering with rapidity of production or increasing the net cost.
These gentlemen have likewise so simplified the continuous system of drawing the wick along as to prevent any loss of cotton. In the next place, the structure of the moulds, properly so called, is new. Instead of being cast, as is usually the case, they are rolled and drawn out, thus giving them smooth surfaces and permitting of their being soldered, are assembled by means of threaded bronze sockets. The engravings between Figs. 3 and 4 show these two modes of fixation. At a may be seen the old method of junction by soldering, and at b the screwing of the moulds into the socket. This machine consists of a box which is alternately heated and cooled, and which is fixed upon a frame, A, at the lower part of which are located the wick bobbins, E. Toward the top of the machine there is a mechanism for actuating the two pairs of jaws, B, which grasp the candles forced upward by the play of the pistons, D. This mechanism, which is controlled by a lever, acts by means of an eccentric.
The pistons, D, are hollow, and are provided above with pieces which form the small end of the candles. Instead of using tin, as is usually done, the Messrs. Barlow employ galvanized iron in the construction of these pistons, and mount them through screw rings—no soldering being used. For this reason, any workman whatever can quickly replace one of the tubes. All the pistons are placed upon a horizontal table, which is made to rise and descend at will, in order to regulate the length of the candles and remove them from the mould. A winch transmits the motion which is communicated to it to two pairs of pinions that gear with racks fixed to the frame to lift the table that supports the pistons. How these latter are mounted may be seen from an inspection of Figs. 3 to 5. This new arrangement of spiral springs for the purpose is designed to hold the pistons on the table firmly, and at the same time to prevent the shock that their upper ends might undergo in case of an abrupt turn of the winch. Moreover, the forged iron plate, H, is not exposed to breakage as it is in other machines, where it is of cast iron. The bobbins already mentioned revolve upon strong iron rods, and the moving forward of the wick in the moulds is effected automatically by the very fact of the manufactured candles' being forced out. These latter are held in position through the double play of the jaws, B, while the stearic acid is flowing into the upper part of the moulds. The cotton wick is thus drawn along and kept in the axis of the candles.
One peculiarity of the machine consists in the waste system applied to the mould box. Steam or hot or cold water is sent into the latter through the conduit, L, starting from a junction between pipes provided with cocks. When the water contained in the box is in excess, it flows out through the waste pipes, G, which terminate in a single conduit. Owing to the branchings at T, and to the cocks of the conduits that converge at L, it is very easy to vary the temperature of the box at will. The warm or cold water or steam may be admitted or shut off simultaneously.
When first beginning operations, the wick is introduced into each mould by hand. The piston table is raised by means of the winch, and is held in this position through the engaging of a click with a ratchet on the windlass. A fine iron rod long enough to reach beneath the pistons and catch the end of the wick is next introduced. After this is removed, the wick is fixed once for all, and in any way whatever, to the top of the mould. This operation having been accomplished, the piston table is lowered, and the machine is ready to receive the stearic acid. The moulds are of tin and are open at both ends. In order to facilitate the removal of the candles, they are made slightly conical. When the candles have hardened, the ends are equalized with a wooden or tin spatula, and then the piston table is raised. At this instant, the jaws, B, are closed so as to hold the candles in place. The latter, in rising, pull into the mould a new length of wick, well centered. A slight downward tension is exerted upon the wick by hand, then a new operation is begun. During this time, the candles held between the jaws having become hard, their wicks are now cut by means of the levers, C, and they are removed from the machine and submitted to a finishing process.—Revue Industrielle.
In several former notes and articles in these pages, we have spoken of the severe crisis through which the old established, or "Leblanc," process has now for some years been passing. It is, in fact, pushed well nigh out of the running by the newer process, known as the "ammonia-soda" process, and would have had to give up the battle before now were it not for the fact that one of its by-products, bleaching powder, cannot, so far, be produced at all by the ammonia-soda works. The bleaching powder trade has thus remained in the hands of the workers of the Leblanc process, and its sale has enabled them to cover much of the loss which they are suffering on the manufacture of soda ash and caustic soda.
In brief outline, the old Leblanc process consists in the following operations: Salt is decomposed and boiled down with sulphuric acid. Sulphate of sodium is formed, and a large amount of hydrochloric acid is given off. This is condensed, and is utilized in the manufacture of the bleaching powder mentioned above. The sulphate of sodium, known as "salt cake," is mixed with certain proportions of small coal and limestone, and subjected to a further treatment in a furnace, by which a set of reactions take place, causing the conversion of the sulphate of sodium of the "salt cake" into carbonate of sodium, a quantity of sulphide of calcium being produced at the same time. The mass resulting from this process is known as "black ash." It is extracted with water, which dissolves out the carbonate of sodium, which is sold as such or worked into "caustic" soda, as may be required. The insoluble residue is the "alkali waste," which forms the vast piles, so hideous to look at and so dreadful to smell, which surround our large alkali works.
The sulphuric acid required for the conversion of the salt into "salt cake" is made by the alkali manufacturer himself, this manufacture necessitating a large plant of "lead chambers" and accessories, and keeping up an immense trade in pyrites from Spain and Portugal. The development of the alkali trade in this country has been something colossal, and the interests involved in it and connected with it are so great that anything affecting it may safely be said to be of truly national importance, quite apart from what technical interest it may possess.
The "ammonia-soda" process, which has played such havoc with the old style of manufacture, proceeds on totally different lines. Briefly stated, it depends on the fact that if a solution of salt in water is mixed with bicarbonate of ammonium, under proper conditions, a reaction takes place by which the salt, or chloride of sodium, is converted at once into bicarbonate of sodium, the bicarbonate of ammonium being at the same time converted into chloride of ammonium.
The bicarbonate of sodium settles out at once as insoluble crystals, easily removed, marketable at once as such, or easily converted into simple carbonate of sodium, and further into caustic soda, as in the ordinary "old" process. The residual chloride of ammonium is decomposed by distillation with lime, giving ammonia for reconversion into bicarbonate of ammonium, and chloride of calcium, which is a waste product.
The maker of "ammonia" soda works direct on the brine, as pumped from the salt fields. His plant is simpler and less costly, and he arrives at his first marketable product much more rapidly and with very much lower working costs than the maker of Leblanc soda, in spite of all the great mechanical improvements which have of late years been introduced into the old process, and which have cheapened its work.
The original patents on the use of ammonium bicarbonate have, we understand, long since expired. But the working details of the process and much of the most successful apparatus have undergone great development and improvement during late years, all the important points being covered by patents still in force, and mainly, if not wholly, in the hands of the one large firm which is now carrying on the manufacture in this country, and is controlling the market.
The one weak spot of the ammonia-soda process, as we mentioned before, is its inability to supply hydrochloric acid or chlorine, and so allow of making bleaching powder. Time after time it has been announced positively that the problem was solved, that the ammonia-soda makers had devised a method of producing hydrochloric acid or chlorine, or both, without the use of sulphuric acid. But the announcements have so far proved baseless, and at present the Leblanc makers are getting incredulous, and do not much excite themselves over new statements of the kind, though they know that if once their rivals had this weapon in their hands the battle would be over and the Leblanc process doomed to rapid extinction.
Such is at present the state of the struggle in this great industry, and the above outline sketch of the two processes is designed to give some idea of the conditions to such of our readers as may not have any special knowledge of these manufactures.
At the present moment great interest is being taken in a new process, about to be put to work on a large scale, which is designed to take up the cudgels against the ammonia process and enable the Leblanc makers to continue the fight on something more like equal terms.
We allude to the process proposed and patented by Messrs. Parnell & Simpson, and about to be worked by the "Lancashire Alkali and Sulphur Company," at Widnes. We recently had the opportunity of inspecting fully the plant erected, and of having the method of procedure explained to us. We look upon the new process as such a spirited attempt to turn the tide of a long and losing battle, and as so very interesting on its own merits, that an account of it in these pages will be thoroughly in place.
The main idea of the process is to combine the "Leblanc" and the "ammonia-soda" manufacture. But in place of using caustic lime to decompose the ammonium chloride and get back the ammonia, the "alkali waste" spoken of above is employed, it being found that not only is the ammonia driven off, but that also the sulphur in the "waste" is obtained in a form allowing of its easy utilization, it and the ammonia combining to form ammonium sulphide, which passes over in gaseous form from the decomposing apparatus. This ammonium sulphide is, as we shall see, quite as available for the working of the ammonia-soda manufacture as pure and simple ammonia, and all the sulphur can be obtained from it.
In outline the process is as follows: We will suppose that a quantity of bicarbonate of sodium has been just precipitated from a brine solution, and we have the residual ammonium chloride to deal with. This is decomposed by "alkali waste," giving a final liquor of calcium chloride, which is run to waste, and a quantity of ammonium sulphide gas. This latter is led at once into a solution of salt in water, till saturation takes place. Into this liquor of brine and ammonium sulphide pure carbonic acid gas is now passed. The ammonium sulphide is decomposed, pure sulphureted hydrogen gas is given off, which is conducted to a gas holder and stored, while ammonium bicarbonate is formed in the liquor, which brings about the conversion of the salt into bicarbonate of sodium, ready for removal and preparation for the market.
It will be observed that we printed the word pure in italics in speaking of the carbonic acid used. This is one of the great points in the process, as in order that the sulphureted hydrogen gas obtained shall be concentrated and pure, only pure carbonic acid can be used in liberating it. The apparatus employed in its preparation is perhaps the most ingenious part of the works, and well worthy of attention by others besides alkali makers. The method is based on the fact that if dilute impure carbonic acid is passed into a solution of carbonate of sodium, the carbonic acid is absorbed, bicarbonate of sodium being formed, and the diluting gases passing away.
The bicarbonate of sodium on heating gives up the extra carbonic acid, which can be collected and stored pure, while the liquor passes back to simple carbonate of sodium, to be used over again as an absorbent. This is not at all new in theory, of course, nor is this the first proposal to use it commercially; but it is claimed that this is the first successful working of it on a large scale.
The gases from a large limekiln supply the dilute carbonic acid gas, which contains 25 per cent. to 30 per cent. of pure gas, the principal diluting gas being, of course, nitrogen. This kiln gas is drawn from the kiln by a blowing engine, and is first cooled in two large receivers. It is then forced into the solution of sodium carbonate in the absorption tower, 65 ft. high by 6 ft. diameter, filled with the liquor. The tower has many diaphragms and perforated "mushrooms," to cause a proper dispersion of the gases as they ascend through the liquor. The strength of liquor found best adapted for the work is equal to a density of about 30° Twaddell. After saturation the mud of bicarbonate of sodium is drawn off and passed into the "decomposer," a tower 35 ft. high by 6 ft. 6 in. in diameter, with perforated shelves, into which steam is blown from below, the liquor passing downward. The bicarbonate is decomposed, pure carbonic acid being given off. This is passed through a scrubber and into a gas holder ready for use. The liquor, which has now returned to the state of simple carbonate of sodium, only requires cooling to be ready to absorb a fresh lot of carbonic acid gas. The cooling is effected in a tower packed loosely with bricks, the hot liquor trickling down against a powerful current of air blown in from below. Liquor has been cooled in this way, in once passing through the tower, from 220° Fahr. to 58° Fahr., but of course the exact cooling obtained depends more or less on the temperature of the atmosphere.
The next stage of the process, if we follow on after the preparation of the pure carbonic acid, is the employment of the gas for the decomposition of the ammonium sulphide absorbed in a brine liquor as above explained. The brine and ammonium sulphide are contained in what is known as a "Solvay tower," provided with proper means for dispersion and absorption of the carbonic acid gas. The precipitated bicarbonate of sodium is removed and washed, and prepared for the market in whatever form is required, the sulphureted hydrogen gas being led to a holder and stored, as before stated.
The decomposition of the ammonium chloride by means of "alkali waste" is carried out in a specially designed still. This is a tower 45 ft. high by 8 ft. diameter, divided by horizontal plates into compartments of about 3 ft. 8 in. in height. These compartments communicate with one another by means of pockets, or recesses, in the shell of the tower. A vertical shaft, with arms, revolves in the tower. The "waste" is fed in at the top by means of hopper and screw feed. The liquor is heated by steam blown in to over 212° Fahr. The ammonium sulphide is led direct into an absorbing vessel full of brine.
It now only remains to see how it is proposed to deal with the sulphureted hydrogen gas which represents the sulphur recovered from the waste. It can be burnt direct to sulphurous acid and utilized for the production of vitriol perfectly pure and free from arsenic, commanding a special price. But Messrs. Parnell & Simpson state that by a method of restricted combustion they are able to obtain nearly all the sulphur as such, and put it on the market on equal terms with the best Sicilian sulphur. We did not gather that this has yet been done on the working scale, however.
It will be seen that it is proposed that a Leblanc alkali maker shall continue to produce a portion of his make by the old process, but shall erect plant to enable him to make another portion by the Parnell & Simpson method, using his Leblanc "waste" in place of the caustic lime now employed by the ammonia soda people. He is thus to have the benefit of the cheaper process for, say, half his make, while he further cheapens the ammonia method by saving the cost of lime and by recovering the sulphur otherwise lost in his waste.
The saving in lime is stated to be one ton for each ton of sodium carbonate produced, or in cash value about 10s. per ton at Widnes, while the sulphur saved is estimated to be 6 cwt. per ton of sodium carbonate. We reproduce these figures with all reserve, not being ourselves sufficiently specialists to judge of them. But we were assured that they represent the minimum expected, and reasons were given to us to show that they would probably be exceeded.
Another gain for the Leblanc maker would be that he will escape the cost of removal and disposal of a portion of his refuse or waste.
The plant now erected was calculated for a yield of one hundred tons carbonate of sodium and about thirty-five tons of sulphur per week, but it now appears likely that this will be exceeded; while the carbonic acid plant was supposed to be equal to a yield of 6 tons of pure gas per day, and is now found capable of doing twice as much.
A few weeks will now bring this new combination process into the active and crucial test of the markets. Chemists and chemical engineers have all along taken a keen interest in the ingenious ideas of Parnell & Simpson. Commercial men are no less interested in the financial result of the experiment about to be tried at the expense of a few gentlemen of Liverpool and district. So far as we can learn, opinions are to some extent divided, though many good judges are very hopefully inclined. For our own part, speaking with diffidence, as being a little off our regular track of work, we will only say that we were favorably impressed with what we saw and heard; and we certainly wish the venture that full success which its cleverness and its pluck, as well as its great importance at this crisis, deserve for it.—Engineering.
An important subject for investigation, which has not yet been satisfactorily determined, is the temperature at which it is most beneficial to distill coals of various qualities. The practice of allowing the charge to remain in the retort for some time after most of the gas has been driven off, to enable (it is said) the retort to recover heat for the next charge, often leads to misconception as to the true temperature of carbonization. The effect of this is to equalize the temperatures inside and outside the retort. This inside temperature is not maintained, the temperature outside not being high enough to transmit the heat with sufficient rapidity; and so, in an apparently hot retort, the coal may be carbonized at a comparatively low temperature. A truer test of temperature is that of the outside of the retort, which should be not less than 400° to 500° Fahr. above the temperature necessary for proper carbonization. In all experiments relating to temperature pretending to any degree of accuracy, a pyrometer of some kind should be used. Judging of the temperature by the color is often misleading. Not only may the eye be deceived, but different clays do not present the same appearance at the same temperature. A good, reliable pyrometer to estimate temperatures to (say) 2500° Fahr. is much wanted.
Experience during the last few years with the high temperatures obtained by the use of regenerative furnaces has led me to the conclusion that higher heats than are usual may be employed with advantage, as regards both the quantity and the quality of gas, provided the retorts are heated uniformly throughout their length, and the weight and duration of the charge are so adjusted that the coal does not remain longer in the retort than is just sufficient to drive off the gas; and that the more rapidly the coal is carbonized, the better are the results. In two retorts of the same size, one making 5,000 and the other 10,000 cubic feet per day, the gas will be twice as long in contact with the surface of the retort in the former as in the latter—to the probable detriment of its quality, and increased tendency to stoppage in the ascension pipes.
A subject closely allied to that just alluded to is the temperature of the gas as it leaves the retort. Until within the last few years, it was generally assumed that this was not higher than from 200° to 300° Fahr.; and a very plausible theory was given to account for such a comparatively low temperature. A discussion which took place a few years ago in the Journal of Gas Lighting showed that at that time opinions on this subject were not unanimous. But the conclusion arrived at seemed to be that the gas was not higher in temperature than that before stated; and if higher temperatures were observed, they were due to the tarry matter in the gas, and were not those of the gas itself. A little reflection is sufficient to show that the existence of gas intimately mixed with tarry matter at a high temperature, without being itself raised to that temperature, is a physical impossibility.
In a paper read to a Continental gas association about a year ago, the writer stated, as the result of many experiments, that unless the temperature in the ascension pipe rises above 480° Fahr., thickening of the tar in the hydraulic main and choking of the ascension pipe will certainly occur. This led me to make a series of experiments, extending over many months, on the temperature of the gas in the ascension pipes at different points and at various times during the charge. The results of these experiments may be of some interest, and may lead to further investigation. The temperatures were taken by mercurial thermometers registering 600° Fahr., except those near the mouthpiece, which were taken by a Siemens water pyrometer. Every care was exercised to insure accuracy; and the instruments were carefully adjusted. At a distance of 18 inches from the mouthpiece, the temperatures varied from an average of 890°, shortly after the retort was charged, to 518° at the end of the charge; at 12 feet distant from the mouthpiece, the corresponding temperature was 444°, falling to 167° at the end of the charge; and at 22 feet, the average temperature varied from 246° at the commencement to 144° at the end of the charge. These are the averages of a number of experiments. In some instances they were considerably above these averages—temperatures over 900° being frequently obtained. This is about the temperature of a low red heat, and is much higher than any I have seen recorded. When the gas was allowed to issue from a hole in the ascension pipe, 1¼ inches in diameter, 18 inches above the mouthpiece, a strip of lead held about an inch from the orifice was freely melted.
In the settings on which these experiments were made, the middle ascension pipe takes the gas from the two central retorts; and it is of interest to note that in this pipe the temperature of the gas 18 inches from the upper retort was found to be 1014° Fahr., and at the point where it entered the hydraulic main it was 440° Fahr. Zinc was freely melted by the gas issuing from a hole 18 inches from the mouthpiece. The temperatures always fall toward the end of the charge; the fall of temperature in the ascension pipe being a good indication that the charge is worked off. They increase with the heat of the retort and with the weight of the charge.
Experiments were also made to ascertain the temperature of the gas in the retort; and for this purpose one of Murrie's pyrometers was used, the action of which depends on the pressure produced by the vaporization of mercury in a malleable iron tube. The end of this tube was first rested on the top of the coal, but not in contact with the retort. It reached about 18 inches into the retort, and therefore was not in the hottest part. In this position the temperature indicated shortly after charging the retort was 1110° Fahr., gradually rising to 1640° Fahr. The end of the tube was then embedded in the coal, when the pyrometer indicated a temperature of 1260° Fahr. within 30 minutes after the retort was charged; gradually rising toward the end of the charge as before. At the time these temperatures were taken, the retorts were each producing 10,000 cubic feet of gas per day. I had no opportunity of testing the accuracy of the statement that, with lower temperatures, there is a tendency to stoppage of the ascension pipes; but with these high temperatures (contrary to what might be expected) there is no trouble from stoppages.
These experiments, so far as they have gone, lead to the conclusion that the temperature of the gas as it is evolved from the coal is not less than 1200° Fahr., and that cooling commences immediately on the gas leaving the retort. The temperatures being far above that of liquefaction, the gases are cooled very rapidly. The temperature of the gas in the ascension pipe depends on the rapidity with which the gas is evolved—that is to say, the greater the quantity produced in a given time, the less effective is the cooling action of the mouthpiece and the ascension pipe; and although I had no opportunity of testing it, I should expect to find that with retorts making from 5,000 to 6,000 cubic feet of gas per day, the maximum temperature in the ascension pipe 18 inches from the mouthpiece will not exceed 400° to 500° Fahr., while with lower heats and lighter charges the temperatures will be still lower. That these temperatures have some effect in causing or preventing stoppage in the ascension pipes there can be no doubt; and it is important that this subject should be thoroughly investigated.
It is of interest to consider what must be the physical condition of the gas at these high temperatures. All the hydrocarbons which are afterward condensed must then be in the condition of gases having various degrees of condensability, mixed with and rendered visible by a cloud of carbon particles or soot. If this soot could be removed from the gas at this stage without reducing the temperature, we should probably have no thick tar or pitch, but only comparatively light-colored oils; and it might possibly lead to an entirely different mode of conducting the process of condensation.
These are a few of the subjects on which it is extremely desirable that we should possess that complete information which can only be obtained by well-directed investigations with different materials and under varying conditions. There are many others in connection with carbonization and purification which might be mentioned; but I think I have said sufficient to show the necessity that exists for more minute investigation and research. Investigations such as are here indicated do not involve any large expenditure of money; but they do require care and intelligence to prevent errors being made. Experiments should not be condemned as defective because the results differ from old-established theories; yet when this does happen, it is in all cases better to suspect the new experiment rather than the old theory, until the results have been fully established.—Wm. Foulis, Journal of Gas Lighting.
The Widnes Alkali Company have recently erected an enormous revolving black ash furnace, which is 30 ft. in length and has a diameter of 12 ft. 6 in. The inside length is 28 ft. 6 in., with a diameter of 11 ft. 4 in. The furnace is lined with 16,000 fire bricks and 120 fire-clay breakers, each weighing 1¼ cwt. The weight of salt cake per charge, i.e., contained in each charge of salt cake, limestone, mud, and slack, is 8 tons 12 cwt. For 110 tons of salt cake charged there are also used about 100 tons of lime mud and limestone and 55 tons of mixing slack. The total amount of salt cake decomposed weekly is about 400 tons, which may be calculated to yield 240 tons of 60 per cent. caustic soda. There is claimed for this massive furnace an economy in iron plate, in expense on the engine power and on fuel consumed, as well as on wear and tear.—Watson Smith, in Industries.
The inauguration of the statue of Philip Lebon, the inventor of lighting by gas, occurred on the 26th of June, at Chaumont, under the auspices of the Technical Gas Society of France. The statue, which we illustrate herewith, is due to the practiced chisel of the young sculptor Antide Pechine, who has perfectly understood his work, and has represented the inventor at the moment at which he observes a flame start from a glass balloon in which he had heated some sawdust. The attitude is graceful and the expression of the face is meditative and intelligent. The statue, which is ten feet in height, was exhibited at the last Salon. It was cast at the Barbedienne works.
It would be impossible to applaud too much the homage that has just been rendered to the inventor of gas lighting, for Philip Lebon, like so many other benefactors of humanity, has not by far the celebrity that ought to belong to him. When we study the documents that relate to his existence, when we follow the flashes of genius that darted through his brain, when we see the obstacles that he had to conquer, and when we thoroughly examine his great character and the lofty sentiments that animated him, we are seized with admiration for the humble worker who endowed his country with so great a benefit.
Lebon was born at Brachay on the 29th of May, 1767. At the age of twenty, he was admitted to the School of Bridges and Roads, where he soon distinguished himself by his ingenious and investigating turn of mind. His first labors were in connection with the steam engine, then in its infancy, and on April 18, 1792, the young engineer obtained a national award of $400 to continue the experiments that he had begun on the improvement of this apparatus.
It was at about the same epoch that Lebon was put upon the track of lighting by gas, during a sojourn at Brachay. He one day threw a handful of sawdust into a glass vial that he heated over a fire. He observed issuing from the bottle a dense smoke which suddenly caught fire and produced a beautiful luminous flame. The inventor understood the importance of the experiment that he had just performed, and resolved to work it further. He had just found that wood and other combustibles were, under the action of heat, capable of disengaging a gas fit for lighting and heating. He had seen that the gas which is disengaged from wood is accompanied with blackish vapors of an acrid and empyreumatic odor. In order that it might serve for the production of light, it was necessary to free it from these foreign products.
Lebon passed the vapor through a tube into a flask of water, which condensed the tarry and acid substances, and the gas escaped in a state of purity. This modest apparatus was the first image of the gas works; and it comprised the three essential parts thereof—the generating apparatus, the purifying apparatus, and the receiver for collecting the gas.
One year afterward, the inventor had seen Fourcroy, Prony, and the great scientists of his epoch. On the 28th of September, 1799, he took out a patent in which he gives a complete description of his thermo lamp, by means of which he produced a luminous gas, while at the same time manufacturing wood tar and pyroligneous or acetic acid. In this patent he mentions coal as proper to replace wood, and he explains his system with a visible emotion and singular ardor. In reading what he has written we are struck with that form of persuasion that does not permit of doubting that he foresaw the future in reserve for his system.
Unfortunately, Lebon could not devote all his time to his discovery. Being a government engineer, without money and fortune, he had to attend to his duties. He went as an ordinary engineer to Angouleme, but he did not forget his illuminating gas, and he strongly regretted Paris, which he termed "an incomparable focus of study." He devoted himself to mathematics and science, he made himself beloved by all, and his mind wandered far from his daily occupation. The engineer in chief soon complained of him, but a committee appointed to investigate the charges that had been made against him affirmed that he was free from any reproach. He was sent back to his post, but war was decimating the resources of France, and the republic, while Bonaparte was in Italy, no longer had any time to pay its engineers. Lebon wrote some pressing letters to the minister, asking for the sums due on his work, but all of them remained without reply. His wife went to Paris, but her applications were fruitless. She wrote herself to the minister the following letter, which exists in the archives of the School of Bridges and Roads:
"Liberty, equality, fraternity—Paris. 22 Messidor, year VII. of the French Republic, one and indivisible—the wife of Citizen Lebon to Citizen Minister of the Interior:
"It is neither alms nor a favor that I ask of you, it is justice. I have for two months been languishing at 120 leagues from my household. Do not, by further delay, force the father of a family, for want of means, to leave a state for which he has sacrificed everything. ... Have regard for our position, citizen. It is oppressive, and my demand is just. There is more than one motive to persuade me that my application will not be fruitless with a minister who makes it a law and duty for himself to be just.
"Greeting and esteem. Your devoted fellow-citizen,
"Madame Lebon, nee De Brambille."
In 1801, Lebon was called to Paris, as attache in the service of Blin, engineer in chief of pavements. He took a second patent—a true scientific memoir full of facts and ideas. It speaks of the numerous applications of illuminating gas and its mode of production, lays down the basis of the entire manufacture—furnaces, condensers, purifiers, gas burners. Nothing is forgotten, not even the steam engine and balloon. Lebon proposed to the government to construct an apparatus for heating and lighting the public buildings, but the offer was rejected. It was then that the unfortunate inventor, wearied by all his tentatives, fatigued by his thousands of vexations, made up his mind to have recourse to the public in order to convince it of the utility of his invention. He rented the hotel Seignelay, St. Dominique-St. Germain St., and invited the public thither. Here he arranged a gas apparatus, which distributed light and heat to all the rooms. He lighted the gardens with thousands of gas jets in the form of rosettes and flowers. A fountain was illuminated with the new gas, and the water that flowed from it seemed to be luminous. The crowd hastened from all parts and came to salute the new invention. Lebon, excited by this success, published a prospectus, a sort of profession of faith, a model of grandeur and sincerity, a true monument of astonishing foresight. He followed his gas into the future and saw it circulating through pipes, whence it threw light into all the streets of future capitals. We reproduce a few passages from this remarkable production:
"It is painful," says he, "and I experience the fact at this moment, to have extraordinary effects to announce. Those who have not seen cry out against the possibility, and those who have seen often judge of the facility of a discovery by what they have to conceive of its demonstration. If the difficulty is conquered, the merit of the inventor vanishes with it. I would rather destroy every idea of merit than allow the slightest appearance of mystery or charlatanism to exist.
"This aeriform principle is freed from those humid vapors that are so injurious and disagreeable to the organs of sight and smell, and of the soot which soils apartments. Purified to perfect transparency, it travels in the state of cold air, and is led by the smallest as well as frailest pipes, by conduits an inch square, formed in the plaster of ceilings or walls, and even tubes of gummed taffety would perfectly answer the purpose. Only the extremity of the tube, which puts the inflammable gas in contact with the air, and upon which the flame rests, should be of metal."
Every one finally paid homage to the illustrious inventor, and a committee appointed in the name of the minister affirmed that "the advantageous results given by the experiments of Citizen Lebon have met and even exceeded the hopes of the friends of the sciences and arts." Napoleon I. soon granted Lebon a concession in the forest of Rouvray for the organization of an industry of wood distillation and gas making. Unfortunately, Lebon was obliged to undertake too many things at once. He prepared the gas, and produced acetic acid and tar that he had to send to Harve for the use of the navy. Despite all his trouble and fatigue, he had something like a ray of hope. He believed that he saw the day of fortune dawning. His works were visited by numerous scientists, and among others the Russian princes Galitzin and Dolgorouki, who, in the name of their government, proposed to the inventor to transfer his plant to Russia, he to be free to set forth the conditions. Lebon refused this splendid offer, and, in an outburst of patriotism, answered that his discovery belonged to his country, and that no other nation should before his own have the benefit of his labors.
The hopes of Lebon were of short duration. Enemies and competitors caused him a thousand troubles, and the elements themselves seemed to turn against him. During a hurricane, the humble house in which he dwelt was destroyed, and a fire shortly afterward consumed a portion of his works. Fatality, like the genius of old, seemed to be following up the unfortunate inventor; but sorrows and reverses could not have any hold on this invincible spirit, who was so well seconded by a wife of lofty character. Lebon, always at work, was seemingly about to triumph over all obstacles, and the hour of the realization of his project of lighting on a large scale was near, when a death as tragic as it was mysterious snatched him from his labors. On the very day of the crowning of the emperor, December 2, 1804, the body of Philip Lebon was found lying inert and lifeless in the Champs Elysees, exhibiting thirteen deep wounds made by a dagger.—La Nature.
The paper I have to lay before you describes the last product of the brain of one of your past presidents—Alexander Angus Croll—in connection with our industry. It may not be so well known to some of the younger as it is to many of the older members of the Institute that the fertile brain of Mr. Croll has done much for the improvement and the extension of the gas industry. I consider that he has been the most successful pioneer both in the cheapening and the purification of gas—two elements without which our industry would progress but slowly if at all; and the success which has crowned his efforts, to our advantage, has reflected itself favorably on himself, showing by his financial success that he has also been a good man of business. All these are conditions which enhance the value of this paper. In the present instance, I claim no other credit than that of being the mouthpiece of Mr. Croll, whose assistant I was for ten of the busiest and most important years of his eventful life; and having (with my son Bruce) taken part in the experiments, I have been asked to describe the process to the Institute.
The manufacture of sulphate of ammonia, as hitherto conducted, has consisted either in bringing together sulphuric acid and ammoniacal liquor or in distilling the liquor by external heat, or by the introduction of steam, and bringing it into contact with the acid in the form of gases and vapor of water. In either case a large volume of noxious gases is given off, the chief of which, being sulphureted hydrogen, has to be fixed by another method, in order to comply with acts of Parliament for the prevention of nuisances.
By the processes hitherto used, we sometimes get only 1¼ tons of salts to every ton of acid used; while in the more perfect forms of apparatus, we may get 1-1/3 tons of salts. By Mr. Croll's process, however, we get an increased yield of salts on the acid used, as follows: The experiments were made with sulphuric acid of the specific gravity of 1838, or nearly concentrated oil of vitriol; and the quantity used was 8 ounces in each experiment. The ammoniacal liquor was of uniform strength throughout all the experiments, being kept in a corked jar; and the solution of sulphate of ammonia was passed through filter paper before being crystallized. Thus we obtained a white salt. In each experiment the solution of sulphate was divided into four equal parts by weight, and one part filtered and crystallized to dryness over a spirit lamp; the weight in each experiment being as nearly as possible the same, or 3¼ oz. of salt to 2 oz. of acid—being in the proportion of 26 oz. of sulphate to 1 lb. of acid, or 32½ cwt. of salts to 20 cwt. of acid.
The results surprised me; and being uniform over a number of experiments, pleased me. Still, I preserved the character of a critic and said: "I should like to treat 8 oz. of acid in the ordinary way—saturating it with ammoniacal liquor, and then crystallizing it." "Oh!" Mr. Croll said, "we know what that will produce." I replied: "Yes; but I would like to do it with the precise acid and liquor we have been using, so that we may have the experiment on all fours with yours, barring your process." These experiments were made at his country residence. I was staying there for the night. So next morning I got down before him, went at my experiment, saturated 8 oz. of acid (and a nice smell I made) out in the grounds, treated it afterward by division into four parts, filtered and crystallized it, all as before, with the result that I obtained 2¾ oz., as against his 3½ oz.—or in the proportion of 27½ cwt. of salt to the ton of acid, as against his 32½ cwt.
I now thought of business. "What is the royalty to be?" I said, as we sat at breakfast. This we settled as we Scotch say "in a crack," or as an Englishman would say "in a jiffy." Mr. Croll decided to have the apparatus put up on a manufacturing scale here in Glasgow; and I determined to erect similar apparatus at one of my gas works.
I dare say that it will be uppermost in your minds, Whence comes the increased yield of salts? Well, I will state one fact, and leave you to ruminate on it, namely, by Mr. Croll's process we did not seem to produce any sulphureted hydrogen. The experiments were conducted in a room with ordinary doors and windows, but without a chimney; and we were not troubled with any offensive smell—a state of things that could not possibly have existed had we been experimenting with any other apparatus hitherto employed in the manufacture of sulphate of ammonia. The apparatus, which will presently be described, only substitutes, for the present mode of distillation, a new one, which forms the subject of Mr. Croll's patent. All other parts of present apparatus can remain as they now exist.
Mr Croll has also introduced another mode of producing sulphate of ammonia, which dispenses with all the apparatus hitherto in use after the distillatory portion, and produces the salt in a state fit for the farmer, ready to be put on the land. This process consists in sending the products of distillation through a vessel filled with wood sawdust saturated with sulphuric acid. The ammonia becomes fixed and crystallized in the sawdust, and is ready for use. There are many works, both at home and abroad, to which the conveyance of sulphuric acid is both difficult and expensive, on account of the cost of carriage and the breakage which occurs; and thus in many such works the ammonia is not utilized. This saturated sawdust process will, I think, remove the difficulty; for I find that dry sawdust absorbs double its own weight of sulphuric acid, and this could be conveyed in the most ordinary casks in a damp state, and save all waste and annoyance from breakage of bottles. In this state it could be used by the farmer, or the sulphate of ammonia could be washed out, crystallized, and exported in the state of salt.
In the remainder of this paper I have been assisted by my son Bruce, who also assisted in the experiments that I have described. He has since been engaged on the trials on a manufacturing scale; and I ask you to permit him to read the concluding portion of the paper, in which he will describe the process, and what he has done.
The process referred to in the foregoing portion of the paper is a method employed for heating the liquor, whereby a chemical action is brought into play, with the results already mentioned. This method consists in passing the products of combustion of a furnace from a clear fire in a hot state through a still containing the ammoniacal liquor. The hot gases from the furnace impart their heat to the liquor, causing the volatilization of the condensed gases, and at the same time act chemically upon the liquor and evolved gases, so that ammonia and sulphuric acid are resulting products, in the compound state of sulphate of ammonia. The formation of the ammonia produced in the process is probably due to the decomposition of nitrogenous bodies contained in solution in the liquor—the sulphocyanide, for instance; the nitrogen being given off in the form of ammonia. Of the sulphuric acid produced, we look upon the sulphureted hydrogen as the source, also any sulphites existing in the liquor, which in their volatile state take up the atom of oxygen necessary for their conversion into sulphate.
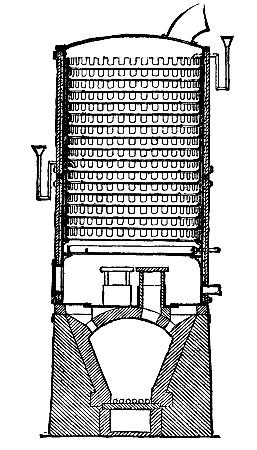
The apparatus used in working the process consists of a tower still, containing a number of superposed trays about 3 or 4 inches apart, with a lipped hole through the bottom of each at the side. The trays are so placed in the tower that the holes are at alternate sides. The liquor passes into the top of the still, and zigzags down through the series of trays, as in an ordinary Coffey still. The bottom tray differs from the rest; being much deeper, and having holes through it connecting it with the furnace, which is set immediately below it. The products of combustion of the fuel are caused to pass from the furnace up through the holes in the trays in the still, and, together with the gases evolved from the liquor, are directed into the saturator, where the sulphate of ammonia is obtained either in solution or in the crystalline state.
Where the process is at present being worked, an exhauster is used to draw the furnace gases through the still; but it might be advantageous to use a blower.
A small plant has been put in action at the gas works in Kilkenny and another on a larger scale, and differing somewhat in detail, here in Glasgow at the Alum and Ammonia Company's works, where the liquor from the Tradeston Gas Works is converted. The trials on a working scale have only been made at both places within the past ten days; and, so far, nothing has appeared against the principle, though in certain of the details of construction some alterations are being made to improve it. The extra yield of salt from a given quantity of acid obtained in the experiments has been proved in practice, as also the absorption of the sulphureted hydrogen.
The other day, while ammoniacal liquor of about 9 oz. strength was being run at the rate of 70 gallons per hour through the still, 5 feet in diameter and 10 feet high, containing seventeen trays, no smell of sulphureted hydrogen was perceptible from the waste gases from the saturator, although on applying lead paper a slight trace of this impurity was noticeable, and it may be stated that the gases were being delivered at the ground level, where there was no difficulty in testing them.
In the Glasgow apparatus we have found it advisable to enlarge the pipe leading the gases into the saturator, as the volume of these is much greater than would be the case in the ordinary method of working. Further experience will probably indicate the desirability of increasing the height of the still, which, being only 10 feet, is not more than half the height that Coffey stills are ordinarily made.
Read at the recent meeting of the Gas Institute, Glasgow.
Whatever may be the position of British pharmacists in comparison with those of other countries, it cannot be said that they have paid the attention to the analysis of urine which the subject has received from pharmacists on the Continent. Considering the importance of the subject, this curious neglect can only be attributed to the fact that the pharmacist in Great Britain is but slowly attaining the position of chemical expert to the physician, which his foreign confrere has so long held with credit and even distinction. In France, for example, M. Méhu, whose name is familiar to readers of this journal, is looked upon as one of the leading authorities on morbid urine and its analysis, and yet a list of goodly pharmaceutical papers shows that, as the medical analyst, he has not forgotten his connection with pure pharmacy.
There are several points about urinary analysis which entitle it to a very high position in the estimation of pharmacists. In the first place, the physician is no more likely to be fonder of the test tube than of the pestle, of analyzing urine than of compounding his own medicines. Leading men in the profession are more and more setting their faces against the dispensing doctor, and there are numbers among them who admit that they succeed no better as analysts than they do as dispensers.
Some old fashioned practitioners trouble themselves very little about their patients' urine, except, perhaps, in respect of sugar and albumen. On the other hand, numbers of leading physicians, including especially those highly educated gentlemen who cultivate a consulting practice, are in the habit of pushing urinary analysis almost to an excess. One well-known specialist of the writer's acquaintance, with an extensive West End practice, makes quantitative determinations of urea, uric acid, and total acidity, in addition to conducting other diagnostic experiments, on every occasion that he interviews his patients. By this means he has accumulated in his case books a mass of data which he considers most valuable as an aid to diagnosis, and through that to successful treatment.
Pharmacists are proverbially neat-handed, as Mr. Martindale would say, and their habit of conducting dispensing operations which involve the dexterous manipulation of very small quantities of material fit them admirably to undertake volumetric and other rapid analytical determinations. Compared with the doctor there is no doubt that in this matter the chemist is facile princeps, and from the nature of their respective occupations such could only have been expected. A few chemists throughout the country lay themselves out to save their local doctors from unwelcome test tube practice, and these almost to a man find it pay. Some charge a handsome fee to patients, and a small one when the analysis comes through the physician. Others find it to their interest to furnish medical men with qualitative reports on sugar or albumen gratuitously. Although this practice has certain obvious drawbacks, if a doctor sends his prescriptions to a chemist, the latter is often willing to gratuitously perform his chemical work. In the present article we propose to describe briefly but fully the methods which have been found of most value in practice.
It is the practice of some physicians to direct the patient to preserve all the urine passed in twenty-four hours, and to forward this in one bottle for analysis. Others, again, merely send a small sample of "morning" and "evening" urine in separate phials, desiring only a comparative report. In the former case the volume should be accurately measured, and the quantity noted either in fluid ounces or cubic centimeters before commencing the analysis. This need not be done if small samples only are received. The color should be noted. It varies greatly, through every shade of yellow and amber to dark brown, with a tinge of green or red, if the coloring matter of bile or blood is present. Also note relative transparency or cloudiness, specific gravity, and reaction, as all these observations are useful in diagnosis. Odor is not quite so important. The specific gravity should be taken at about 60° F. in an ordinary specific gravity bottle, or more conveniently by means of a good urinometer. In the latter case it is very important to have an instrument of known accuracy, many of those in the market being valueless. Urinometers of glass, though fragile, are decidedly more cleanly and less liable to get out of order than the gilded brass instruments carried in the pocket by many physicians. Mr. J.J. Hicks, of 8 Hatton Garden, E.C., manufactures a very creditable "patent urinometer" at an extremely low cost. Healthy urine has a density of from 1.015 to 1.025; but variations from this range are common.
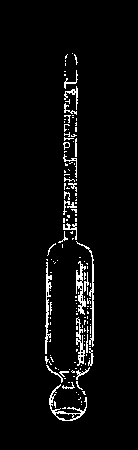
A fair quantity of the urine, after shaking, should be placed in a tall conical glass vessel, to allow easy collection of the precipitate for subsequent, microscopical examination. If an abundant amorphous deposit of a fawn or pink—from uroerythrin—color slowly settles and is readily diffused, urates in excess can be anticipated. Their presence is proved by the readiness with which they dissolve on warming with the supernatant urine to about the temperature of the blood. No difficulty is experienced if small quantities of albumen are present, as that body is not coagulated until the temperature rises much higher. A sandy precipitate of free uric acid will not dissolve on warming the urine, and its identity can further be determined by means of the microscope, or by applying a well-known color-reaction. A grain or so is oxidized into reddish alloxan and alloxantin by carefuly evaporating with a few drops of strong nitric acid on a piece of porcelain. A little ammonia is then added, when the fine purple murexide stain will be produced.
It is always advisable to mention the reaction to test papers of all samples received. Urine is normally acid, but there are certain diseases which render fluid neutral or alkaline. The urea of acid urine on standing is changed by a putrefactive ferment into ammonic carbonate, but this decomposition in a state of health should not take place for at least twenty-four hours. Alkalies, or organic salts of alkaline metals, when taken as medicine render the urine alkaline, and the indication is then not of much moment; but if none of these causes exist, the condition is of serious diagnostic import. Where it is desired to determine the degree of acidity of the urine voided, say, by a gouty patient, a dilute volumetric solution of caustic soda should be employed, using a few drops of an alcoholic solution of phenolphthalein as an indicator, and reporting in terms of oxalic acid. The soda solution may conveniently contain the equivalent of one milligramme of recrystallized oxalic acid (H2C2O4.2H2O) in each cubic centimeter.
Carbamide, as it is called by systematic chemists, or urea, is next to water the largest constituent of urine, and forms about one-third of its total solids. Derived from ammonic carbonate by abstracting two molecules of the elements of water, it is readily converted by putrefaction into that salt, and the urine under these circumstances becomes strongly alkaline in reaction. Earthy phosphates then fall naturally out of solution, so that the putrid fluid is always well furnished with sediment. Nitrogen that has served its purpose as muscle or other proteid leaves the animal economy chiefly in the form of urea, and its proportion in the urine, therefore, is a fair index of the activity of wasting influences.
For its determination Knop's sodic hypobromite method, on account of its convenience, is now generally preferred. The volumetric process of Liebig, which depends on the formation of an insoluble compound of urea with mercuric nitrate, possesses no advantages and is troublesome to work. The principle of the hypobromite process is simple. In a strongly alkaline solution urea is broken up by sodic hypobromite, its nitrogen being evolved in the gaseous state, and its carbon and hydrogen oxidized to carbonic anhydride and water respectively. The volume of free nitrogen obtained bears a direct ratio to the amount of urea decomposed.
Among the number of instruments which have been introduced for the purpose of conveniently measuring the evolved gas, that of Gerrard, an illustration of which we give, is one of the simplest, cheapest, and best. The ureometer tube, b, is connected at the base with a movable reservoir, c, and by means of a rubber tube passing through a cork at the top to the generating bottle, a. To use the apparatus, fill b to zero with water and have the reservoir placed so high that it contains only an inch or so of the liquid. Replace the cork with attached tube tightly in b. Now pour into the generating bottle 25 c.c. of a solution prepared by dissolving 1 part of caustic soda in 2½ parts of distilled water, and dexterously break in the liquid a tube containing 2.2 c.c. of bromine. The tubes will be found very convenient, obviating entirely the suffocating fumes diffused in the act of measuring bromine. Allow to stand in the solution of sodic hypobromite thus prepared a test tube containing exactly 5 c.c. of the urine under examination. Cork the bottle as shown in the illustration, see that the water is at zero, and that the liquid in the reservoir is at the same level, and then allow the urine to gradually mix with the hypobromite solution. Cool the evolved gas by placing the bottle in cold water, adjust the levels of the water in the tube and reservoir (to obviate a correction for pressure), and read off the percentage of urea in terms of which the tube is graduated. Stale urine, the urea of which has largely been converted into ammonic carbonate, still yields a very fair result, that salt being also completely split up by the powerful oxidant employed. Should the urine contain albumen, it is advisable to remove it by boiling and filtering, as, although only slowly decomposed by the hypobromite solution, it communicates to the liquid such a tendency to froth that the disengagement of the nitrogen is seriously impeded. Most of those alkaloids which might possibly be present do not yield the gas when treated in this manner, and therefore may be disregarded.
Glucose, so characteristic of diabetes mellitus, is not difficult of detection or estimation. The facility with which it reduces alkaline cupric, argentic, bismuthous, ferric, mercuric salts, indigo and potassic picrate and chromate solutions has been utilized for the preparation of several ready methods for its determination. Trommer's test consists in adding enough cupric sulphate to color green, then excess of alkali, and boiling. Yellow to brick-red cuprous oxide forms as a heavy precipitate if glucose is present. The organic matter of the urine prevents the precipitation of cupric hydrate on the addition of the alkali. This test is delicate and deservedly popular. Fehling's well-known solution contains sodio-potassic tartrate, which serves the purpose chiefly of retaining the copper in solution. Unfortunately, Fehling's original solution has a tendency to become hyper-sensitive if kept long, a proneness to change that is much increased on dilution. When so altered, the solution will yield a more or less copious precipitate of cuprous oxide on merely boiling, and quite independent of the presence of glucose. This decomposition is obviated by preserving the copper salt in a separate solution from the tartrate and alkali, and mixing before use. Schmiedeberg substitutes mannite and Cresswell glycerin for the Rochelle salt, in order to render the solution stable. Some prepared by the writer over twelve months ago, according to the suggestion of the latter physician, has since shown no signs of decomposition, and is now as good as it was then. For qualitative purposes the solution may be prepared thus: Dissolve 35 gm. of recrystallized cupric sulphate and 200 c.c. of pure glycerin in 100 c.c. of distilled water. Dissolve separately 80 gm. of caustic soda in 400 c.c. of water. Mix the solutions and boil for a quarter of an hour. A small amount of reduction from impurity in the glycerin takes place. Allow to stand till clear, decant, and dilute to 1,250 c.c. Ten cubic centimeters will then equal roughly 5 centigrammes of glucose. For exact quantitative determination it is necessary to standardize the solution with pure anhydrous dextrose.
To a practiced operator the indications yielded by the use of this test are of great value; but beginners are exceedingly liable to mistake its various reactions, and to report the urine as saccharine when normal traces only of sugar are present. The bismuth test of Bottger, as greatly improved by Nylander, is fairly delicate, and not so easily misread as Fehling's. A large volume of reagent being used with a comparatively small quantity of urine, the precipitate of earthy phosphates does not interfere in the least with the reaction. On boiling about 3 drachms of Nylander's solution and 20 minims of urine for a minute or two, the liquid darkens with a trace of sugar, and becomes opaque and black if the latter is present in quantity. The reagent is prepared by dissolving 494 grains of caustic soda, 247 grains of Rochelle salt, and 154 grains of subnitrate of bismuth (free from silver) in 13 fluid oz. of distilled water. It should be decanted for use from any sediment.
In those cases where the amount of glucose present is required to be determined, Dr. Pavy's ammonia cupric process distances all compeers for ease of application and delicacy of end-reaction, combined with considerable accuracy. His solution differs from that of Fehling in containing ammonia, which dissolves the cuprous oxide as soon as it is formed, yielding a colorless solution. It is only necessary, therefore, to note the moment that the blue color of the liquid is exactly discharged, in order to tell when all the copper present has been reduced. Pavy's solution is prepared as follows: Dissolve 356 grains of Rochelle salt and the same weight of caustic potash in distilled water; dissolve separately 73 grains of recrystallized cupric sulphate in more water with heat. Add the copper solution to that first prepared, and when cold add 12 fluid oz. of strong ammonia (sp. gr. 0.880), and distilled water to 40 fluid oz. The estimation is thus conducted: Dilute 10 c.c. of the ammoniated cupric solution—equivalent to 5 milligrammes of glucose—with 20 c.c. of distilled water, and place in a 6 or 8 oz. flask. Attach this by means of a cork to the nozzle of an ordinary Mohr's burette, b, preferably fitted with a glass stopcock, and filled previously with the diluted urine. The small tube, c, which traverses the cork is intended to permit the escape of steam. Now raise the blue liquid in the flask to active ebullition—not too violent—by the aid of a spirit lamp or small Bunsen flame. Turn the stopcock in order to allow the urine to flow into the boiling solution at the rate of about 100 drops per minute (not more or much less) until the azure tint is exactly discharged. Then stop the flow, and note the number of cubic centimeters used. That amount of dilute urine will contain 5 milligrammes of glucose. To render the determination as accurate as possible, the urine should be diluted to such an extent that not less than 4 or more than 7 c.c. are required to decolorize the solution, and the proportions necessary will be found to vary from 1 part of urine in 2½ to 1 in 30 or 40. The subsequent calculation is very simple. If you wish to give the percentage of sugar, multiply 0.005 by 100, and divide the product by the number of cubic centimeters of dilute urine employed. The figure thus obtained, multiplied by the extent of dilution—i.e., if there is 1 of urine in 10, multiply by 10—gives the required percentage. The number of grains per fluid ounce can of course be obtained by multiplying the percentage by 4.375. To observe easily the exact end-reaction a piece of white paper should be placed behind the flask. If the analyst objects to the escape of the waste ammoniacal fumes, they may be conducted by a suitable arrangement into water or dilute acid. In addition to glucose there are small quantities of other copper-reducing bodies present in all urine, which always render the reading higher than strict accuracy would demand. Their aggregate proportion, however, is, comparatively speaking, so minute that for most medical purposes their presence may be disregarded. Greater care must be exercised, though, in those instances where such a deoxidizer as chloral hydrate is accidentally present. In case of doubt, a little washed and pressed yeast should be allowed to stand with the urine for a day or two in a warm place. Alcoholic fermentation with evolution of carbonic acid gas soon sets in, and the specific gravity of the liquid is lowered considerably. This reaction points conclusively to the presence of sugar.
Based upon Braun's potassic picrate test, Dr. G. Johnson has devised a colorimetric process for the estimation of sugar. On boiling an alkaline solution of that salt with glucose, the former is reduced to deep red-brown picramate, the color of the liquid, of course, varying in intensity according to the proportion of sugar present. This solution is diluted till it corresponds in tint with a ferric acetate standard, and the percentage of sugar is then readily calculated. For those who prefer this process the convenient apparatus manufactured by Mr. Cetti, of 36 Brooke street, Holborn, is recommended, who will also furnish full particulars of the test.
Normal urine is free from coagulable proteids, though it is admitted that albumen may sometimes occur in the absence of disease. It is always highly important, therefore, to determine accurately the presence or absence of this body. In the relentless malady named after Richard Bright, the urine always contains albumen, and if accompanied by the "casts" of the uriniferous tubules your report may amount to a sentence of certain death. The tests which we now describe are accurate and easily applied; but reliance should never be placed on any single reaction—at any rate until the operator has acquired considerable experience.
Galippe's picric acid test has within the last few years attracted much attention, chiefly through the commendation it has received from Dr. George Johnson. A saturated solution is prepared by dissolving 140 grains of recrystallized picric acid (carbazotic acid, or, more correctly, trinitrophenol) in 1 pint of water with heat, and decanting the clear solution. Some of the urine is rendered perfectly bright by filtration—repeated, if necessary—through good filtering paper, and to this an equal volume of the picric acid solution is added. In the presence of albumen a more or less distinct haze is produced, which on heating to the boiling point is rather intensified than otherwise. Peptones, if present, yield a similar haze, and quinine or other alkaloid a more or less crystalline precipitate; but in both these cases the opalescence is completely dissipated by heat. Mucin, an important constituent of some urines, is not affected by picric acid, and the test is decidedly one of great value.
The nitric acid test. Heller's contact method, which can also be used with the last-described reagent, is the best mode of applying the old-fashioned and favorite test with nitric acid. To 5 volumes of a filtered saturated solution of magnesic sulphate, prepared by dissolving 10 parts of the salt in 13 parts of distilled water, add 1 volume of strong nitric acid, and label "Sir W. Roberts' nitric acid reagent." A couple of drachms of bright filtered urine is allowed to float on an equal quantity of this solution in a test tube; care being taken that the contact line is sharply defined. In a period of time varying from a few seconds to a quarter of an hour, according to the amount of albumen present, a delicate opalescent zone forms at the point of junction, and if mucin also is present, a more diffused haze higher up in the urine. Special attention should be given to the position of the opacity. In some concentrated urines a belt of urates will appear at the line of demarkation; but these dissolve on warming. Moreover, owing to the dilution necessary in the mode of applying Galippe's picric acid test, they are not so readily shown by the latter. A ½ oz. glass syringe can very conveniently be substituted for a test tube in making analyses according to Heller's method. Some of the urine should be drawn up, and then an equal volume of the reagent. On setting aside, the albumen ring will rapidly develop.
The boiling test. This method also is very delicate and valuable. It depends on the well-known property possessed by many proteids of coagulating under the influence of heat. The urine should have an acid reaction to test paper; if alkaline, it must be cautiously neutralized with dilute acetic acid. In either case a single drop of strong acetic acid should be added to about three drachms of the bright liquid. If this precaution is omitted, there is danger of precipitating earthy phosphates on heating; and should a great excess of acid be employed, a non-coagulable form of albumen known as syntonin is formed, besides increasing the likelihood of precipitating mucin. Place the prepared urine in a narrow test-tube and hold it in a small flame so that the upper part only of the liquid approaches the boiling point. By this means very small traces of albumen are easily observed, the opalescence produced contrasting strongly with the cold and clear fluid beneath.
The ferrocyanide test. Hydroferrocyanic acid yields a precipitate immediately in the presence of much albumen, and if traces only are present, in the course of a few minutes. To apply the test, strongly acidulate with acetic acid, and then add a few drops of recently prepared potassic ferrocyanide solution. This is one of the most delicate tests known.
It is often desirable that the percentage of albumen present should be determined at frequent intervals, in order to note the success or otherwise of the physician's treatment. These quantitative determinations, being intended only for comparative purposes, do not demand any very excessive degree of accuracy, such as would be difficult to obtain in ordinary practice. The recent method of a Continental worker. Dr. Esbach, affords indications sufficiently precise for therapeutical requirements, and is at the same time extremely easy of application. The filtered acid urine is poured into the glass tube up to the mark U, and then the special reagent is added till the level of the liquid stands at R.
Mix the liquids thoroughly, without shaking, by reversing the tube a dozen times, close with a cork, and allow it to stand upright for twenty-four hours. The height at which the coagulum then stands, read off on the scale, will indicate the number of parts per thousand, or grammes of albumen in one liter. This divided by ten gives the percentage. Dr. Esbach's test solution is prepared by dissolving 10 grammes of picric acid and 20 grammes of citric acid in 900 c.c. of boiling distilled water, and then adding, when cold, sufficient water to yield 1 liter. The citric acid is only employed for the purpose of maintaining the acidity of the liquid, and is really not essential.
The determination of the proportion of uric acid in urine was formerly rather neglected by physicians. There is now, however, a growing tendency in a certain class of diseases to attach considerable importance to its accurate estimation, and, as some little trouble is involved, pharmacists should be prepared to undertake the work. A rough way is to concentrate somewhat, acidulate with hydrochloric acid, and collect and weigh the precipitate thrown down on standing. There are several objections, however, to this method, and many attempts have been made to elaborate a more reliable process. One of the most recent, and which has been pronounced the most practical and successful, has been devised by Professor Haycraft. Although apparently rather detailed and elaborate, the determination is easy and extremely simple.
The following solutions must be prepared: 1. Dissolve 5 grammes of nitrate of silver in 100 c.c. of distilled water, and add ammonia until the precipitate first formed redissolves. 2. Dilute strong nitric acid with about two volumes of distilled water; boil, to destroy the lower oxides of nitrogen, and preserve in the dark. 3. Dissolve about 8 grammes of ammonic thiocyanate (sulphocyanide) crystals in a liter of water, and adjust to decinormal argentic nitrate solution, by diluting till one volume is exactly equal to a volume of the latter. Dilute the solution thus prepared with nine volumes of distilled water, and label "Centinormal ammonic-thiocyanate solution." 4. A saturated solution of ferric alum. 5. Strong solution of ammonia (sp. gr. 0.880). The uric acid estimation is conducted as follows: Place 25 per cent. of urine in a beaker with 1 gramme of sodic bicarbonate. Add 2 or 3 c.c. of strong ammonia, and then 1 or 2 c.c. of the ammoniated silver solution. If, on allowing the precipitate caused by the latter reagent to subside, a further precipitate is produced by the addition of more solution, the urine contains an iodide, and silver solution must be added till there is an excess. The gelatinous urate must now be collected, the following special procedure being necessary: Prepare an asbestos filter by filling a 4 oz. glass funnel to about one-third with broken glass, and covering this with a bed of asbestos to about a quarter of an inch deep. This is best managed by shaking the latter in a flask with water until the fibers are thoroughly separated, and then pouring the emulsion so made in separate portions on to the broken glass. On account of the nature of the precipitate and of the filter, it is necessary to use a Sprengel pump, in order to suck the liquid through. The small apparatus sold to students by chemical instrument makers will answer the purpose admirably. Having collected the precipitate of silver urate on the prepared filter, wash it repeatedly with distilled water, until the washings cease to become opalescent with a soluble chloride. Now dissolve the pure urate by washing it through the filter with a few cubic centimeters of the special nitric acid. The process is carried out thus: Add to the liquid in the beaker a few drops of the ferric-alum solution to act as an indicator, and from a burette carefully drop in centinormal ammonic thiocyanate until a permanent red coloration of ferric thiocyanate barely appears. The number of cubic centimeters used of the thiocyanate solution multiplied by 0.00168 gives the amount of uric acid in the 25 c.c. One milligramme may be added to compensate for loss, and the whole multiplied by four gives the percentage of uric acid in the urine. The whole process depends on the fact that argentic urate fails to dissolve in ammonia, but is soluble in nitric acid, and is thus easily obtained in the pure state. By determining the amount of combined silver, the percentage of uric acid can readily be calculated. The addition of sodic bicarbonate prevents the otherwise inevitable reduction of the silver salt.
In diseases affecting the liver, the urine frequently becomes contaminated with biliary constituents. If the coloring matter of bile is present (bilirubin, etc.), the liquid is darkened considerably in tint, and may assume various shades of brown or green. Should the color be decided, the fluid will be found to foam strongly on shaking, and white blotting-paper will be stained by it yellow or greenish. These characters point to the presence of bile in fair quantity, and it is only necessary to apply a single confirmatory test. Allow some of the urine to flow carefully, according to Heller's method, over a couple of drachms of yellow nitric acid (i.e., acid containing traces of the lower oxides of nitrogen). A number of rapidly changing colors soon appear, passing through green, blue, violet, and red to yellow. The first of these tints, green, is the only one that undoubtedly points to the presence of biliary coloring matter, all the others being yielded by another constituent of urine, indican, when similarly treated. Should the color of the urine suggest the presence of only traces of bile, the best plan is not to treat the urine directly, but extract a quantity of it by shaking with chloroform. On separating the latter, and covering with yellowish nitric acid, the color changes will be observed penetrating into the chloroform. A little, also, evaporated on a slide yields reddish crystals, which exhibit a pretty play of colors under the microscope when touched with nitric acid.
It is not unfrequently considered important to test urine for the sodium salts of the conjugate biliary acids, taurocholic and glycocholic. Dr. Oliver, of Harrogate, has proposed the use of an acidulated peptone solution for this purpose, and the reaction is undoubtedly a good one. The reagent is prepared by dissolving 30 grains of flesh peptone, 4 grains of salicylic acid, and 30 minims of strong acetic acid, in sufficient water to produce 8 fluid oz. of solution. Thus prepared, the peptone shows no signs of decomposition on keeping. To use the test, mix 1 fluid drachm of the reagent with 20 minims of urine, previously diluted to a standard specific gravity of 1.003. A haze is produced, which will be found to be more or less distinct, according to the proportion of bile salts present.
A normal and variable constituent of urine, chlorine, is not usually required to be determined. Should the estimation be considered necessary, however, Volhard's silver process, which has been noticed in treating of uric acid, possesses several advantages over other methods: 10 c.c. of urine are diluted with 60 c.c. of distilled water. To this is added 2 c.c. of pure 70 percent. nitric acid and 15 c.c. of a standard solution of silver nitrate (1 c.c. = 0.01 gramme NaCl). Shake well and make up to 100 c.c. with water. All the chlorine present will now be precipitated in the liquid as a silver salt. Filter an aliquot part (about 70 or 80 c.c.), and determine in the clear solution the excess of silver with standard ammonic thiocyanate, using the ferric alum indicator. The difference between this and the amount of silver originally present in the aliquot part has been precipitated as silver chloride (AgCl). The whole estimation should be conducted as rapidly as possible. A simple calculation will then give the proportion of chlorine in the dilute urine, and this multiplied by ten shows the percentage. It is usual to report in terms of NaCl.
In those cases where the pharmacist is asked to determine phosphoric acid quantitatively, the uranic-acetate method described in Sutton's "Volumetric Analysis" yields the most satisfactory results. The process requires some little experience to use it with ease, and is too lengthy for quotation here.
A good microscope is one of the first necessaries of the urinary analyst. By its aid it is possible to distinguish easily many solid constituents of urine—normal and pathological; indeed, the examination of urinary deposits is often quite as important as the more elaborate wet analysis. A well-made instrument is no luxury to the pharmacist; but even those whose chief aim is bon marché can procure capital students' microscopes at exceedingly low cost. One of the cheapest, and at the same time an instrument of good quality, is the "Star," manufactured by Messrs. R. & J. Beck, of 31 Cornhill, E.C.
Equipped with a good microscope, the analyst should obtain a fair supply of typical slides for comparison. The following selection will be found sufficient for his purpose: A set of the chief varieties of uric acid, calcic oxalate, and triple phosphate; the urates and oxalurates; urea nitrate, calcic hippurate and carbonate, hippuric acid, cystin, well mounted "casts" of the tubili uriniferi, spermatozoa, etc. In doubtful cases microchemical reagents can be employed, using Professor Attfield's "Chemistry" as a guide. Where mounted objects are not at hand, reference may be made to the capitally executed plates in that work. After obtaining a little experience in the use of the microscope, no difficulty will be met with in these examinations.—The Chemist and Druggist.
All who have learned a little of chemistry doubtless remember the experiment with vortex rings produced by phosphorus trihydride mixed with a little phosphide of hydrogen. As this curious phenomenon evidently does not depend upon the peculiar properties of this gas, I have been trying for some time to reproduce it by means of tobacco smoke, and even with chemical precipitates, which are, in a way, liquid smoke. After a few tentatives made at different times, my experiment succeeded perfectly. The following is, in brief, the mode of operating:
Take up a little hydrochloric acid in a pipette and put a few drops of it into a very dilute solution of nitrate of mercury, and you will obtain rings of mercurial chloride that will, in their descent, take on the same whirling motion that characterizes the aureolas of phosphureted hydrogen.
The drops of acid should be allowed to fall slowly, and from a feeble height, to the surface of the liquid contained in the vessel. It is unnecessary to say that the result may be obtained through the use of other solutions, provided that a precipitate is produced that is not very thick, for in the latter case the rings do not form. If need be, we may have recourse to milk, and carefully pour a few drops of it into a glass of water.
As regards smoke rings, it is easy to produce these by puffing cigar smoke through a tube (Fig. 1). But, in order to insure success, a few precautions are necessary. The least current of air must be avoided, and this requires the closing of the windows and doors. Moreover, in order to interrupt the ascending currents that are formed in proximity to the body, the operation should be performed over a table, as shown in the figure. The rings that pass beyond the table are not perceptibly influenced by currents of hot air. A tube ¾ inch in diameter, made by rolling up a sheet of common letter paper, suffices for making very beautiful rings of one inch or more in diameter. In order to observe the rings well, it is well to project them toward the darkest part of the room, or toward the black table, if the operator is seated. The first puffs will not produce any rings if the tube has not previously been filled with smoke. The whirling motion is perfectly visible on the exit of the ring from the tube, and even far beyond.
As for the aspect of the rings projected with more or less velocity to different distances from the tube, Figs. 2, 3, and 4 give quite a clear idea of that. Figs. 3 and 6 show the mode of destruction of the rings when the air is still. There are always filaments of smoke that fall after being preceded by a sort of cup. These capricious forms of smoke, in spreading through a calm atmosphere, are especially very apparent when the rays of the sun enter the room. Very similar ones may be obtained in a liquid whose transparency is interfered with by producing a precipitate or rings in it.—La Nature.
To the Editor of Scientific American Supplement:
In your issue for August 13 is "A Proposition for a Government Breeding Farm for Cavalry Horses," by Lieutenant S.C. Robertson U.S.A., First Cavalry. The article is national in conception, deep in careful thought, which only gift, with practical experience with ability, could so ably put before the people. As a business proposition, it is creditable to an officer in the United States army.
The husbandman and agriculturist, also the navy and scientific explorations, each in turn present their wants before the government for help in some way, and receive assistance. The seaman wants new and improved or better ships, and the navy gets them; but the poor cavalryman must put up with any kind of a craft he can get; the horse is the cavalryman's ship—war vessel on land.
The appeal of Lieut. Robertson to our government for better horses is reasonable; and he tries to help the government with a carefully studied business proposition through which to enable our government to grant the supplication of the army. That Lieut. Robertson loves a horse, and knows what a good one is, no man can dispute who has read his article; but as to how it can best be produced, he does not know. While I for one applaud both his article and his earnestness, with your permission I will make some suggestions as to the breeding side of his proposition. The business portion will, of course, come under the ordnance department in any event.
As for a government breeding establishment for any kind of livestock in this great agricultural country, I feel that such would be at variance with the interests of husbandry in America.
The breeding of horses is particularly an important branch of agriculture, and the farmers should be assisted by the government in the improvement of their horses, until they are raised to a standard which in case of emergency could supply the army at a moment's notice with the best horses in the world at the least possible expense.
Our government Agricultural Bureau is constantly spending thousands of dollars to help the agriculturist in matter of better and greater varieties of improved seeds and the better way for cultivation. Now, the seed of animal life is as important as in vegetable life to the interest and welfare of the husbandman, which also means the government. For the government to become a monopolist of any important branch in agriculture is not in harmony with the principles of our republican-democratic form of government. While advocating a protective tariff against outside depreciation of home industries, our government should not in any way approach monarchical intrusion upon the industries of its husbandmen. Our government cannot afford to make its agriculturists competitors in so important a matter to them (the farmers) as in the raising of horses; but the government can see to it that the husbandman has a standard for excellence in the breeding of horses which shall be recognized as a national standard the civilized world over. Then, by that standard, and through our superior advantages over any other civilized nation in the vast extent of cheap and good grass lands, with abundance of pure water, and with all temperatures of climate, we can grow, as a people, the best horses in the world, to be known as the National Horse of America. Our government must have a blood standard for the breeding of horses, by which our horses can be bred and raised true to a type, able to reproduce itself in any country to which we may export them; and the types can be several, as our territory is so great and demands so varied, but blood and breeding must be the standard for each type. Our fancy breeders have a standard now, called a "time standard," which is purely a gambling standard, demoralizing in all its tendencies to both man and beast. With this the government need have nothing to do, for it will die out of itself as the masses learn more of it, and especially would it cease to be, once the government established a blood standard for the breeding of all horses, and particularly a National Horse.
When the cereal crops of our country are light, or the prices fall below profitable production, the farmer has always a colt or two to sell, thus helping him through the year. In place of constantly importing horses from France, England, and Scotland, where they are raised mostly in paddocks, and paying out annually millions of dollars, it is our duty to be exporting.
As an American I am ashamed when I see paraded at our county or state fairs stallions and mares wearing the "blue ribbon" of superexcellence, with boastful exclamation by the owner of "a thoroughbred imported Percheron, or a thoroughbred imported French coacher, or a thoroughbred imported Scotch Clyde, or a thoroughbred imported English coacher, or a thoroughbred imported English Shire, or a thoroughbred imported English Cleveland Bay!"
The American farmer and his boys look on aghast at the majesty and beauty of these prize winners over our big-headed, crowbar-necked, limp-tailed, peeked-quartered horses called "standard bred!" What standard? "Time standard," as created by a man who is neither a horseman nor a breeder; but because of the lack of intelligent information and want of courage upon the part of a few, this man's ipse dixit has become law for the American breeders until such time as cultured intelligence shall cause them to rebel. It soon will.
It is indeed time for the government to step in and regulate our horse breeding. Of all the national industries there is none of more importance than that of horses. More so in America than in any other country, because our facilities are greater, and results can be greater under proper regulation. Lieut. Robertson has proved to be the right man in the right place, to open the door for glorious results to our nation. No one man or a small body of men can regulate this horse-breeding industry, but as in France, Russia, and England, the government must place its hand and voice.
We are indeed an infant country, but have grown to an age where parental restraint must be used now, if ever. We have millions of farmers in America, breeding annually millions of horses; and except we have another internal war, our horses will soon become a burden and a pest.
There are numbers of rich men throughout the country breeding fancy horses, for sport and speculation, but they only add to the increasing burden of useless animals, except for gambling purposes; for they are neither work horses, coach horses, nor saddle horses. Our farmers of the land are the breeders, as our recent war of the rebellion testified. The war of 1812, the Mexican war of 1847, and the war of 1861 each called for horses at a moment's notice, and our farmers supplied them, destroying foundation bloods for recuperation. From 1861 to 1863 the noble patriotism of our farmers caused them to vie with each other as to who should give the best and least money to help the government; and cannot our government now do something for the strength and sinew of the land, the farmers?
I was dealing in horses, more or less, from 1861 to 1863 (as I had been before and long after), and many was the magnificent horse I saw led out by the farmer for the government, at a minimum price, when, previous to 1861, $400, $500, and even $600 was refused for the same animals. Horses that would prove a headlight to any gentleman's coach in the city, and others that would trot off fourteen to sixteen miles an hour on the road as easy as they would eat their oats, went into the cavalry or artillery or to baggage trains. What were left for recuperation at the close of the war were mongrels from Canada or the Indian and wild lands of the West, and such other lazy brutes as our good farmers would not impose upon the government with or later were condemned by the army buyers. These were largely of the Abdallah type of horse, noted for coarseness, homeliness, also soft and lazy constitutions. No one disputes the brute homeliness of the Abdallah horse, and in this the old and trite saying of "Like begets like" is exemplified in descendants, with which our country is flooded. The speed element of which we boast was left in our mares of Arabian blood through Clay and Morgan, but was so limited in numbers as to be an apology for our present time standard in the breeding of fancy horses. Knowing that Abdallah blood produced no speed, and being largely ignorant as to the breeding of our mares, which were greatly scattered over the land after the war, some kind of a guess had to be made as to the possibility of the colts we were breeding, hence the time standard fallacy. But it has ruined enough men, and gone far enough.
Upon Lieutenant Robertson's proposition, a turn can be made, and a solid base for blood with breeding of all American horses can be demanded by the government for the country's good.
From the earliest history of man, as a people increased in wealth, they gave attention to mental culture with refinement; following which the horse was cultivated to a high blood standard with national pride. From the Egyptians, the Moors, the Romans, and Britons to France, Russia, and Prussia we look, finding the horse by each nation had been a national pride—each nation resorting to the same primitive blood from which to create its type, and that primitive was the Arabian. Scientists have theorized, men have written, and boys have imagined in print, as to some other than the Arabian from which to create a type of horse, and yet through all ages we find that Arabian has been the one stepping stone for each advanced nation upon which blood to build its national horse.
Scientists have reasoned and explored, trying to prove to the contrary, but what have they proved? The Arabian horse still remains the fact.
The lion, the tiger, the leopard, still remain the same, as does the ass and the zebra. As God created and man named them, with all animal life, subject to the will of man, so do they all continue to remain and reproduce, each true to its type, free from imperfections or disease; also the same in vegetable and mineral life. In animal life, the build, form, color, size, and instincts remain the same, true to its blood from the first, and yet all was created for man through which to amuse him and make him work.
It is a fact that all of man's creations from any primitive life, either animal or vegetable, will degenerate and cease to be, while of God's perfect creations, all continue the same.
We will condense on the horse. The Arabian is the most pliable in its blood of any other known to man. From it, any other type can be created. Once a type has been created, it must be sustained in itself by close breeding, which can be continued for quite a number of years without degeneracy. For invigoration or revitalizing, resort must be made to its primitive blood cause. To go out of the family to colder or even warmer creations of man means greater mongrelization of both blood and instinct, also to invite new diseases.
Nothing is more infatuating than the breeding of horses. A gifted practical student in the laws of animal life may create a new and fixed type of horse, but it can be as quickly destroyed by the multitude, through ignorant mongrelization.
In the breeding of horses, our people are wild; and in no industry can our government do more good than in making laws relating to their breeding. It can father the production of a national horse without owning a breeding farm. It can make blood and breeding a standard for different types, and see to it that its laws are obeyed, thus benefiting all the agriculturists, and have breeding farms in America; and also itself as a government, financially. We must not however begin upon the creation of other nations, but independently upon God's gift to man, as did England, France, and Russia. That a government should interfere in the breeding of horses is no new thing. The Arabs of the desert boast to this day of King Solomon's stud of horses; but in each and every instance where a nation has regulated and encouraged the breeding of the horse to a high standard of excellence, they have all begun at the primitive, or Arabian. Thus England in boasting of her thoroughbred race horse admits it to be of Arabian origin. Russia in boasting of her Orloff trotting and saddle horse tells you it is of Arabian origin. France boldly informs you that her Percheron is but an enlarged Arabian, and offers annual special premiums to such as revitalize it with fresh Arabian blood.
After the war of 1812 our forefathers imported many Arabian stallions to recuperate the blood of their remnants in horses. From 1830 such prominent men as Andrew Jackson and Henry Clay said all they could by private letter and public speech to encourage the importation of and breeding freely to the Arabian horse, and specially did the State of Kentucky follow the advice of Henry Clay, so that from 1830 up to 1857 Kentucky had more Arabian stallions in her little district than the combined States of the Union. Kentucky has had a prestige in her mares since the war, and it comes in the larger amount of Arabian blood influence she has had in them, than could be found elsewhere. Kentucky is shut in, as it were, and retaining her mares largely impregnated with Arabian blood, all that was necessary for them to do was to get trotting-bred stallions from New York State, then eclipse all other States in the produce. While I cheerfully award to Kentucky all credit due to it, I am not willing that Lieut. Robertson should make his base for government breeding establishment sectional, nor would I submit to England through Kentucky. I am too American for that.
For cavalry purposes, the Prussian horse is the best in the world, and is also Arabian in its closest foundation.
To get at this blood question more definitely, let us inquire into these different recognized self-producing national types of horses abroad.
First is the English thoroughbred race horse, which is simply an improved Arab. The functions of this English national horse are but twofold—to run races and to beget himself, after which he ceases to be of value. He is not a producer of any other type of value; to breed him out of his family is mongrelism and degeneracy, so we don't want him, even though we could humiliate our American pride through our loved State of Kentucky.
Count Orloff of Russia was a great horseman, exceedingly fond of horseback riding independent of the chase. He tried in 1800 to breed a satisfactory horse from the English thoroughbred race horse, but went from bad to worse until he resorted to the ever-pliant blood of the Arabian. He sent to Egypt and secured a thoroughbred Arabian stallion, paying $8,000 for him (in our money). This horse he bred to Danish mares, largely of Arabian blood, and created a very stout, short-backed horse, standing from 15½ to 15¾ and 16 hands high, of great trotting speed, also able to run to weight, and with good disposition, which the English thoroughbred did not have. This type he continued to close-breed, going back to the Arabian for renewed stoutness. At his death, his estates passed to his daughter, who continued her father's breedings until the Russian government purchased the entire collection, about 1846, since when the Russian government Orloff trotting and saddle horse has become famous the world over as a first-class saddle, cavalry, stage coach, and trotting horse combined. They are broken at three years of age, and scarce any that cannot beat 2:30 at trotting speed, and from that down to 2:15 in their crude way of hitching and driving. This is something for American breeders to think very interestedly upon.
France wanted heavy draught horses, also proud coach horses; so rather than go to any competing nation for their created types, her enterprising subjects took the same Arabian blood, and from it created the beautiful Percheron, also French coach horses, so greatly valued and admired the world over, and which the gifted and immortal Rosa Bonheur has so happily reproduced upon canvas. Can America show any kind of a horse to tempt her brush?
With regard to a foundation for a government or national horse, I am certain so gifted and able United States officer as Mr. S.C. Robertson did not know that it was unnecessary to go to England for the blood of their national horse, even though we smuggled it through Kentucky or any other of our States. Again, it would be impossible to produce any type of a horse from the English thoroughbred, except a dunghill, and Mr. Robertson would not have his government breed national dunghills!
I love England as our mother country, but am an American, born and dyed in the wool to our independence, from the "Declaration."
Now let us see what England says of her thoroughbred: "He is no longer to be relied upon for fulfilling his twofold functions as a racer and reproducer of himself. He is degenerating in stoutness and speed. As a sire he has acquired faults of constitution and temper which, while leaving him the best we have, is not the best we should aspire to have. His stoutness and speed are distinctly Arabian qualities, to which we must resort for fresh and pure blood." We have shown that the Englishman says "his thoroughbred is full of radical and growing defects in wind, tendons, feet, and temper, and that his twofold functions are to run races and reproduce himself, which are the end of his purpose." Does our government want breeding farms upon which to nurse these admitted "defects," including the "confirmed roarer," for cavalry horses? I quote again: "Those who have had most to do with him are ready to admit that he no longer possesses the soundness, stoutness, speed, courage, and beauty he inherited from his Arabian parentage. As a sire for half-bred stock, he may do for those who will use him, but we must resort to the Arabian if we would revitalize and sustain our thoroughbred race horse."
In the face of these statements, in print abroad, would Lieut. Robertson make the base for our proposed national horse that of the English thoroughbred, scattering the weeds from such imperfect breedings among the farmers of our land?
I am writing as an old horseman and breeder, and not as a newspaper man or young enthusiast, although the enthusiasm of youth is still in me, for which I am thankful.
This question of horse breeding I have been deeply interested in for forty years past. Let me quote to the reader from one of many letters I have received from Sir Wilfrid Seawen Blunt during the past seven years. His practical knowledge of the English thoroughbred race horse and his blood cause, the Arabian, is the equal if not superior to any other one man of this present age.
With his wife, Lady Anne, he dwelt with the different tribes of the desert, studying the Arabs as a people, in their customs and habits, also traditions with beliefs. In matter of their horses, Mr. Blunt made a special study, while Lady Anne put her diaries in book form after her return, and which book should be owned by every cultured and educated lady in America. After spending a year in Arabia, traveling both sides of the Euphrates and through Mesopotamia, as no other Anglo-Saxons have been known to do, living with the different Bedouin tribes of the desert as they lived, Mr. Blunt and his wife, Lady Anne, came out with sixteen of the choicest bred mares to be found, also two stallions, the mares mostly with foal. These were placed upon their estates, "Crabbet Park," to continue inbreeding as upon the desert, pure to its blood. As this question in itself will make a long and interesting article, I will avoid it at present, quoting to the reader from one of my old letters:
"CRABBET PARK, SUSSEX, ENGLAND.
"Dear Sir: Political matters have prevented an earlier reply to your last.
"I am well satisfied with my present results, and shall not abandon what I have undertaken. The practical merits of Arabian blood are well understood by us.
"Our sale of young stock maintains itself in good prices in spite of bad times; indeed, my average within the past two years has risen from £84 to £102 on the pure-breds sold as yearlings, and we receive the most flattering and satisfactory accounts from purchasers, although it is known that I retain the best of each year's produce, and so have greatly improved my breeding stock.
"You speak of the opinions of the press as against you. The sporting press are not breeders, but are the mouthpiece of prejudices. We have had them somewhat against us, but they now view things in more friendly tone.
"For immediate use in running races (in which the sporting press are chiefly interested), the Arabian in his undeveloped state and under size will not compete with the English race horse. This fact has caused racing men to doubt his other many and more important merits; indeed, it is only those who have had personal experience of him that as yet acknowledge them.
"The strong points in the Arabian are many:
"First, his undoubted soundness in constitution, in wind, limb, and feet. It will be noticed that the Englishman must have soundness in wind, limb, and feet, showing that their thoroughbred is the thorn in that particular. The Arabian has also wonderful intelligence, great beauty, and good disposition, with an almost affectionate desire to adapt himself to your wishes.
"In breeding, I have found the pure-breds delicate during the first few weeks after birth, and have lost a good many, especially those foaled early in the year; yet it is a remarkable fact that during the eight years of my breeding them, I have had no serious illness in the stables; once over the dangerous age, they seem to have excellent constitutions, and are always sound in wind, limb, and feet.
"Second, they are nearly all good natural and fast walkers, also fast trotters; and from the soundness of their feet are especially fitted for fast road work, being able to do almost any number of miles without fatigue.
"Third, they are nearly all good natural jumpers, and I have not had a single instance of a colt that would not go across country well to hounds.
"They are very bold fencers, requiring neither whip nor spur. They carry weight well, making bold and easy jumps where other larger horses fail.
"Fourth, they have naturally good mouths, and good tempers, with free and easy paces; so that one who has accustomed himself to riding a pure-bred Arabian will hardly go back, if he can help it, to any other sort of horse.
"There is all the difference in riding the Arabian and the ordinary English hunter or half-bred that there is in riding in a well-hung gig or a cart without springs.
"Fifth. As sires for half-bred stock, the Arabian may not be better than a first-class English thoroughbred, but is certainly better than a second-class one, and first-class sires are out of the reach of all ordinary breeders; for that reason I recommend a fair trial of his quality, confident your breeders will not be disappointed.
"With good young mares who require a horse to give their offspring quality, that is to say, beauty, with courage and stoutness, and with a turn of speed for fast road work, the Arabian is better than any class of English thoroughbreds that are used for cross breeding.
"I trust then for that reason you will not allow yourself to be discouraged by the slowness of the people to appreciate all the merits of the Arabian at once.
"Our breeders are full of prejudices, and only experience can teach them the value of things outside their own circle of knowledge.
"I have no doubt whatever that truth will in the end prevail; but you must have patience. Remember that a public is always impatient, and most often unreasonably so.
"My stud I keep at a permanent strength of twelve brood mares, and as many fillies growing in reserve.
"You ask me regarding the pacing gait. I have seen it in the pure-bred Arabs on the desert; and in many parts of the East it is cultivated, notably in Asia Minor and Barbary. The walk, pace, amble, trot, and run are found in the Arabian, and either can be cultivated as a specialty.
"If you think any of my letters to you are of general value to your people, I am quite willing you should so use them.
"I am, very truly yours,
"WILFRID SCAWEN BLUNT.
"To RANDOLPH HUNTINGTON, Rochester, N.Y."
My experience with Arabian blood the past seven years justifies all that Mr. Blunt has predicted to me from time to time. So also do old letters by Andrew Jackson and Henry Clay hold out the same inducements to the breeders of Kentucky and Tennessee in their day.
From my long years of experience in all classes of horses, I am frank to say to-day that I would not be without a thoroughbred Arabian stallion on my place, and journalists who inform their readers that they "are liable to splints, ringbones, and spavins," give themselves away to all intelligent readers and breeders as exceedingly superficial in matter of horses; for ringbones and spavins are positively unknown among the Arabs. The way to get rid of such imperfections in our mongrel breed of horses is to fill them up with pure Arab blood.
Such paper men also talk about "fresh Diomed" and "fresh Messenger blood," as though there had been a drop of it in never so diluted form for any influence these many years, of course forgetting that Diomed was a very strongly inbred Arabian horse. He came to this country when 21 years old.
He was foaled 1777, and arrived in Virginia in 1798. From his old age and rough voyage in an old-fashioned ship, it required nearly a year to recuperate from the journey, and was 23 years old before he could do stud service to any extent. Then, at no time to his death was he a sure foal getter, even to a few mares. He died in 1808, thirty-one years old, long enfeebled and unfit for service.
Between 1808 and 1887 is quite a period of time, during which we have had four different wars, beginning with 1812, and how much Diomed blood does the reader suppose there is in this country? Yet I take up daily and weekly papers devoted to horse articles, extolling the value of Diomed blood as cause for excellence in some young horse. Are we a nation of idiots to be influenced by such nonsense?
I wish there was fresh Diomed blood; thus the public would know what Arab blood had done for England. So I can say of imported Messenger. What our breeders want is good, solid information in print, and not the; dreamings of some professional writer for money. For myself, I am on the downhill side of life, but so long as I can help the young by pen or example, I shall try.
RANDOLPH HUNTINGTON.
Rochester, N.Y.
In the province of the Rhine there is a range of mountains, including several extinct volcanoes, which offer grand and beautiful scenery and every opportunity for geological study, leading the mind back to the early ages of the earth.
Let us take an imaginary trip through this region, starting on our wanderings from the Rhine, where it breaks through the vine-clad slate mountains of the Westerwald and the Eifel. A short distance above the mouth of the Ahr we leave its banks, turning to the west, and entering the mountains at the village of Nieder Breisig. A pretty valley leads us up through orchards and meadows. The lower hills are covered with vineyards and the mountains with a dense growth of bushes, so that we do not obtain an extended view until we reach an elevated ridge.
 DISTANT VIEW OF THE VOLCANIC PORTION OF THE EIFEL, TAKEN FROM THE HEIGHTS OF THE SCHNEIFEL.
DISTANT VIEW OF THE VOLCANIC PORTION OF THE EIFEL, TAKEN FROM THE HEIGHTS OF THE SCHNEIFEL.
The valley of the Rhine lies far below us, but the glittering surface of the river, with the little towns, the castles and villas and the gardens and vineyards on its banks are still visible, while in the background the mountains of the Westerwald have risen above the hills on the river. This range stretches out into a long wooded ridge crowned by cone-shaped peaks of basalt. To the northwest of this lies Siebengebirge, with its numerous domes and pinnacles, making a grand picture veiled in the blue mist of distance. On the opposite side we have a very different view of curious dome and cone shaped summits surrounded by undulating plateaus or descending into deep ravines and gorges. It is the western part of the volcanic region of Rhineland which lies before us, and in the center of which is the Laachersee or lake of Laach. The origin of these volcanoes is not as remote as many suppose, but their activity must have continued for a comparatively long period, judging from the extent of their lava beds.

There was a time when the sea covered the lowlands of North Germany, and the waves of a deep bay washed the slopes of the Siebengebirge. Then the bed of the Rhine lay in the highlands, which it gradually washed away until the surface of the river was far, far below the level of its old bed; and then the volcanoes poured forth their streams of lava over the surrounding plains.
In the course of time the surface of the country has changed so that these lava beds now lie on the mountain sides overhanging the valleys of to-day. Some of the volcanoes sent forth melted stones and ashes from their summits, and streams of lava from their sides, while the craters of others cracked and then sank in, throwing their debris over the neighboring country. In the Eifel there are many such funnels which now contain water forming beautiful lakes (Maaren), which add much to the scenery of the Eifel. The Laachersee is the largest of these lakes. In the mean time the channel of the Rhine had been worn away almost to its present level, but the mountains still sent forth their streams of lava, which stopped brooks and filled the ravines, and even the Rhine itself was dammed up by the great stream from Fornicherkopf forming what was formerly the Neuwied. The old lava stream which obstructed the river is still to be seen in a towering wall of rock, extending close beside the road and track that follow the shore.

After having made these observations, we descend from the height which afforded us the view of the Vinrt Valley. A clear brook flows through green meadows and variegated fields stretch along the mountain sides, while modest little villages are scattered among the fruit trees. On the other side of the valley rises the Herchenberg, an extinct volcano. As we climb its sides we see traces of the former devastation. Loose ashes cover the ground, bits of mica glittering in the sun, and on the summit we find enormous masses of stone which were melted and then baked together. In the center lies the old crater, a quiet, barren place bearing very little vegetation, but from its wall an excellent view of the surrounding country can be obtained. Not far from this mountain lies the mighty Bausenberg, with its immense, well preserved crater, only one side of which has been broken away, and which is covered with a thick growth of bushes. The ledges of this mountain are full of interest for the mineralogist. Nearer to Lake Laach are the Wahnenkopfe, the proud Veitskopf, and other cone-shaped peaks. To these we direct our steps, and after a long tramp over the rolling, cultivated plateau, we climb the wood-covered sides of the great basin in whose depths the Laachersee lies. From the shore of this lake rise the high volcanic peaks which tower above all the other mountains.

Tired from our climb through the ashes, which are heated by the sun, we rest in the shade of a beech-wood, looking through the leaves into the valley below us, with the old cloisters and the high Roman church which the monks once built on the banks of the lake.

To the south of the lake rise other volcanoes, lying on the border of the fertile Maifeld, which gradually descends to the valley of Neuwied. Here, at the southern declivity of the group of volcanoes which surrounds the Laachersee, remarkably large streams of lava were ejected, covering the surface of the plateau with a thick layer. The largest of these streams is that from the Niedermendig, which consists of porous masses of nepheline lava. In the time of the Romans millstones were made from this mass of rock, and the industry is carried on now on a larger scale. It is a strange sight which meets one's eyes when, after descending through narrow passages, he finds himself in large, dark halls, from which the stone has been cut away, and in which there are well-like shafts. The stones are raised through these shafts by means of gigantic cranes and engines. Because of the rapid evaporation of the water in the porous stone, these vaults are always cool, winter and summer, and therefore they are used by several brewers as storehouses for their beer, which owes its fame to these underground halls.

Although the traces of former volcanic action are evident to the student of nature, the Rhine with its mild climate and luxuriant vegetation has covered many marks of the former chaotic state of the land. Very little of this beauty is seen on the higher and, therefore, more severe and barren mountains of the Western Eifel, through which a volcanic fissure runs from the foot of the high unhospitable Schneifel to Bertrich Baths, near the Moselle. From the ridge of the Schneifel the traveler from the north has his first glimpse of the still distant system of volcanoes. The most beautiful part of this portion of the Eifel is in the neighborhood of Dann and Manderscheid. Near the former rises a barren mountain with a long ridge, on each side of which is a deep basin. These are sunken craters, which now contain lakes, and near these two there is a third, larger lake, the Maar von Schalkemehren, on the cultivated banks of which we find a little village. The middle one, the Weinfelder Maar, is the most interesting for geologists, for there seems to have been scarcely any change here since the time of the eruption. On the other side of the mountain lies the Gremundener Maar, the shores of which are not barren and waste land, like those of the middle lake, but it is surrounded by a dark wreath of woods whose tops are mirrored in the crystal water. Farther to the south, near the villages of Gillenfeld and Meerfeld, there are more lakes.

The grandest picture of these ancient events is offered by the Mosenberg, near Manderscheid, a mighty volcano which commands an extensive view of the country. Two old craters lie on its double top, one of which has fallen in, forming a short rocky valley, but the other retains its original regular shape. In the circular funnel, whose walls consist of masses of lava stone, rests a quiet, black lake, that looks very mysterious to the wanderer. Only low juniper bushes grow near the crater, bearing witness to the barrenness of the land. From the foot of this mountain an immense stream of lava, as wide and deep as a glacier, broke forth and flowed into the valley, where the end of the stream is still to be seen in a high, steep wall of rock.
 THE "CHEESE GROTTO" AT BERTRICH BATHS.
THE "CHEESE GROTTO" AT BERTRICH BATHS.
Similar sights are met all through this western volcanic region, and we can consider the mineral and acid springs, which are very numerous, as the last traces of the former disturbances, the products of the decomposition of the volcanic stones buried in the earth. At Bertrich Baths there are hot springs which were known to the Romans, for numerous antiquities dating from their time have been excavated here. Near these springs, at Bertrich, there is a "Cheese Grotto," which is a break through the foot of a stream of lava, the stones of which have not assumed the usual form of solidified columns, but have taken flat, round shapes which resemble the forms of cheeses.
Now we have completed our wanderings, which required only a few days, although they extended over this whole volcanic region, and which end here on the Moselle.—Ueber Land und Meer; Allgemeine Illustrirte Zeitung.
[Nature.]
The Meteorological Institute at Upsala has gained so much fame by the investigations on clouds which have been carried on there during the last few years, that a few notes on a recent visit to that establishment will interest many readers.
The Institute is not a government establishment; it is entirely maintained by the University of Upsala. The personnel consists of Prof. Hildebrandsson, as director; M. Ekholm and one other male assistant, besides a lady who does the telegraphic and some of the computing work.
The main building contains a commodious office, with a small library and living apartments for the assistant. The principal instrument room is a separate pavilion in the garden. Here is located Thiorell's meteograph, which records automatically every quarter of an hour on a slip of paper the height of the barometer, and the readings of the wet and dry thermometers. Another instrument records the direction and velocity of the wind.
This meteograph of Thiorell's is a very remarkable instrument. Every fifteen minutes an apparatus is let loose which causes three wires to descend from rest till they are stopped by reaching the level of the mercury in the different tubes. When contact is made with the surface of the mercuries, an electric current passes and stops the descent of each wire at the proper time. The downward motion of the three wires has actuated three wheels, each of which carries a series of types on its edge, to denote successive readings of its own instrument. For instance, the barometer-wheel carries successive numbers for every five-hundredth of a millimeter—760.00, 760.05, 760.1, etc.; so that when the motion is stopped the uppermost type gives in figures the actual reading of the barometer. Then a subsidiary arrangement first inks the types, then prints them on a slip of paper, and finally winds the dipping wires up to zero again.
An ingenious apparatus prevents the electricity from sparking when contact is made, so that there is no oxidation of the mercury. The mechanism is singularly beautiful, and it is quite fascinating to watch the self acting starting, stopping, inking, and printing arrangements.
We could not but admire the exquisite order in which the whole apparatus was maintained. The sides of the various glass tubes were as clean as when they were new, and the surfaces of the mercuries were as bright as looking glasses.
The university may well be proud that the instruments were entirely constructed in Stockholm by the skillful mechanic Sorrenson, though the cost is necessarily high. The meteograph, with the anemograph, cost £600, but the great advantage is that no assistant is required to sit up at night, and that all the figures wanted for climatic constants are ready tabulated without any further labor.
But the Institute is most justly celebrated for the researches on the motion and heights of clouds that have been carried on of late years under the guidance of Prof. Hildebrandsson, with the assistance of Messrs. Ekholm and Hagström.
The first studies were on the motion of clouds round cyclones and anticyclones; but the results are now so well known that we need not do more than mention them here.
Latterly the far more difficult subjects of cloud heights and cloud velocities have been taken up, and as the methods employed and the results that have been obtained are both novel and important, we will describe what we saw there.
We should remark, in the first instance, that the motion of the higher atmosphere is far better studied by clouds than by observations on mountain tops, for on the latter the results are always more or less influenced by the local effect of the mountain in deflecting the wind and forcing it upward.
The instrument which they employ to measure the angles from which to deduce the height of the clouds is a peculiar form of altazimuth that was originally designed by Prof. Mohn, of Christiania, for measuring the parallax of the aurora borealis. It resembles an astronomical altazimuth, but instead of a telescope it carries an open tube without any lenses. The portion corresponding to the object glass is formed by thin cross wires: and that corresponding to the eye piece by a plate of brass, pierced in the center by a small circular hole an eighth of an inch in diameter. The tube of the telescope is replaced by a lattice of brass work, so as to diminish, as far as possible, the resistance of the wind. The vertical and horizontal circles are divided decimally, and this much facilitates the reduction of the readings.
The general appearance of the instrument is well shown in the figure, which is engraved from a photograph I took of Mr. Ekholm while actually engaged in talking through a telephone to M. Hagström as to what portion of a cloud should be observed. The latticework tube, the cross wires in place of an object glass, and the vertical circle are very obvious, while the horizontal circle is so much end on that it can scarcely be recognized except by the tangent screw which is seen near the lower telephone.
Two such instruments are placed at the opposite extremities of a suitable base. The new base at Upsala has a length of 4,272 feet; the old one was about half the length. The result of the change has been that the mean error of a single determination of the highest clouds has been reduced from 9 to a little more than 3 per cent. of the actual height. At the same time the difficulty of identifying a particular spot on a low cloud is considerably increased. A wire is laid between the two ends of the base, and each observer is provided with two telephones—one for speaking, the other for listening. When an observation is to be taken, the conversation goes on somewhat as follows: First observer, who takes the lead—"Do you see a patch of cloud away down west?" "Yes." "Can you make out a well-marked point on the leading edge?" "Yes." "Well, then; now." At this signal both observers put down their telephones, which have hitherto engaged both their hands, begin to count fifteen seconds, and adjust their instruments to the point of cloud agreed on. At the fifteenth second they stop, read the various arcs, and the operation is complete.
But when the angles have been measured the height has to be calculated, and also the horizontal and vertical velocities of the cloud by combining the position and height at two successive measurements at a short interval. There are already well-known trigonometrical formulæ for calculating all these elements, if all the observations are good; but at Upsala they do far more. Not only are the observations first controlled by forming an equation to express the condition that the two lines of sight from either end of the base should meet in a point, if the angles have been correctly measured and all bad sets rejected; but the mean errors of the rectangular co-ordinates are calculated by the method of least squares.
This figure shows the peculiar ocular part of the altazimuth, with the vertical and horizontal circles. It also shows the telephonic arrangement.
The whole of the calculations are combined into a series of formulæ which are necessarily complicated, and even by using logarithms of addition and subtraction and one or two subsidiary tables—such as for log. sin²(θ/2) specially constructed for this work—the computation of each set of observations takes about twenty minutes.
Before we describe the principal results that have been attained, it may be well to compare this with the other methods which have been used to determine the height of clouds. A great deal of time and skill and money have been spent at Kew in trying to perfect the photographic method of measuring the height of clouds. Very elaborate cloud cameras, or photo-nephoscopes, have been constructed, by means of which photographs of a cloud were taken simultaneously from both ends of a suitable base. The altitude and azimuth of the center of the plate were read off by the graduated circles which were attached to the cameras; and the angular measurements of any point of cloud on the picture were calculated by proper measurements from the known center of the photographic plate. When all this is done, the result ought to be the same as if the altitude and azimuth of the point of the cloud had been taken directly by an ordinary angle measuring instrument.
It might have been thought that there would be less chance of mistaking the point of the cloud to be measured, if you had the pictures from the two ends of the base to look at leisurely than if you could only converse through a telephone with the observer at the other end of the base. But in practice it is not so. No one who has not seen such cloud photographs can realize the difficulty of identifying any point of a low cloud when seen from two stations half a mile or a whole mile apart, and for other reasons, which we will give presently, the form of a cloud is not so well defined in a photograph as it is to the naked eye.
At Kew an extremely ingenious sort of projector has been devised, which gives graphically the required height of a cloud from two simultaneous photographs at opposite ends of the same base, but it is evident that this method is capable of none of the refinements which have been applied to the Upsala measures, and that the rate of vertical ascent or descent of a cloud could hardly be determined by this method. But there is a far greater defect in the photographic method, which at present no skill can surmount.
We saw that the altazimuth employed at Upsala had no lenses. The meaning of this will be obvious to anyone who looks through an opera glass at a faint cloud. He will probably see nothing for want of contrast, and if anything of the nature of a telescope is employed, only well-defined cloud outlines can be seen at all. The same loss of light and contrast occurs with a photographic lens, and many clouds that can be seen in the sky are invisible on the ground glass of the camera. Cirrus and cirro-stratus—the very clouds we want most to observe—are always thin and indefined as regards their form and contrast against the rest of the sky, so that this defect of the method is the more unfortunate.
But even when the image of a cloud is visible on the focusing glass, it does not follow that any image will be seen in the picture. In practice, thin, high white clouds against a blue sky can rarely be taken at all, or only under exceptional circumstances of illumination. The reason seems to be that there is very little light reflected at all from a thin wisp of cirrus, and what there is must pass through an atmosphere always more or less charged with floating particles of ice or water, besides earthy dust of all kinds. The light which is scattered and diffused by all these small particles is also concentrated on the sensitive plate by the lens, and the resulting negative shows a uniform dark surface for the sky without any trace of the cloud. What image there might have been is buried in photographic fog.
In order to compare the two methods of measuring clouds, I went out one day last December at Upsala with Messrs. Ekholm and Hagström when they were measuring the height of some clouds. It was a dull afternoon, a low foggy stratus was driving rapidly across the sky at a low level, and through the general misty gloom of a northern winter day we could just make out some striated stripes of strato-cirrus—low cirro-stratus—between the openings in the lower cloud layer. The camera and lens that I use habitually for photographing cloud forms—not their angular height—was planted a few feet from the altazimuth with which M. Ekholm was observing, and while he was measuring the necessary angles I took a picture of the clouds. As might have been expected under the circumstances, the low dark cloud came out quite well, but there was not the faintest trace of the strato-cirrus on the negative. MM. Ekholm and Hagström, however, succeeded in measuring both layers of cloud, and found that the low stratus was floating at an altitude of about 2,000 feet high, while the upper strato-cirrus was driving from S. 57° W. at an altitude of 19,653 feet, with a horizontal velocity of 81 and a downward velocity of 7.2 feet per second. This is a remarkable result, and shows conclusively the superiority of the altazimuth to the photographic method of measuring the heights of clouds.
Whenever opportunity occurs, measures of clouds are taken three times a day at Upsala, and it may be well to glance at the principal results that have been obtained.
The greatest height of any cloud which has yet been satisfactorily measured is only 43,800 feet, which is rather less than has usually been supposed; but the highest velocity, 112 miles an hour with a cloud at 28,000 feet, is greater than would have been expected. It may be interesting to note that the isobars when this high velocity was reported were nearly straight, and sloping toward the northwest.
The most important result which has been obtained from all the numerous measures that have been made is the fact clouds are not distributed promiscuously at all heights in the air, but that they have, on the contrary, a most decided tendency to form at three definite levels. The mean summer level of these three stories of clouds at Upsala has been found to be as follows: low clouds—stratus, cumulus, cumulo-nimbus, 2,000-6,000 feet; middle clouds—strato-cirrus and cumulo-cirrus, 12,900-15,000 feet; high clouds—cirrus, cirro-stratus, cirro-cumulus, 20,000-27,000 feet.
It would be premature at present to speculate on the physical significance of this fact, but we find the same definite layers of clouds in the tropics as in these high latitudes, and no future cloud nomenclature or cloud observations will be satisfactory which do not take the idea of these levels into account.
But the refinements of the methods employed allow the diurnal variations both of velocity and altitude to be successfully measured. The velocity observations confirm the results that have been obtained from mountain stations—that, though the general travel of the middle and higher clouds is much greater than that of the surface winds, the diurnal variation of speed at those levels is the reverse of what occurs near the ground. The greatest velocity on the earth's surface is usually about 2 p.m.; whereas the lowest rate of the upper currents is about midday.
The diurnal variation of height is remarkable, for they find at Upsala that the mean height of all varieties of clouds rises in the course of the day, and is higher between 6 and 8 in the evening than either in the early morning or at midday.
Such are the principal results that have been obtained at Upsala, and no doubt they surpass any previous work that has been done on the subject. But whenever we see good results it is worth while to pause a moment to consider the conditions under which the work has been developed, and the nature and nurture of the men by whom the research has been conducted. Scientific research is a delicate plant, that is easily nipped in the bud, but which, under certain surroundings and in a suitable moral atmosphere, develops a vigorous growth.
The Meteorological Institute of Upsala is an offshoot of the Astronomical Observatory of the university; and a university, if properly directed, can develop research which promises no immediate reward in a manner that no other body can approach.
If you want any quantity of a particular kind of calculation, or to carry on the routine of any existing work in an observatory, it is easy to go into the labor market and engage a sufficient number of accurate computers, either by time or piece work, or to find an assistant who will make observations with the regularity of clockwork.
But original research requires not only special natural aptitudes and enthusiasm to begin with, but even then will not flourish unless developed by encouragement and the identification of the worker with his work. It is rarely, except in universities, that men can be found for the highest original research. For there only are young students encouraged to come forward and interest themselves in any work for which they seem to have special aptitude.
Now, this is the history of the Upsala work. Prof. Hildebrandsson was attached as a young man to the meteorological department of the astronomical observatory, and when the study of stars and weather were separated, he obtained the second post in the new Meteorological Institute. From this his great abilities soon raised him to the directorship, which he now holds with so much credit to the university. M. Ekholm, a much younger man, has been brought up in the same manner. First as a student he showed such aptitude for the work as to be engaged as assistant; and now, as the actual observation and reduction of the cloud work is done by him and M. Hagström, the results are published under their names, so that they are thoroughly identified with the work.
Upsala is the center of the intellectual life of Sweden, and there, rather than at Stockholm, could men be found to carry out original research. It redounds to the credit of the university that it has so steadily supported Prof. Hildebrandsson, and that he in his turn has utilized the social and educational system by which he is surrounded to bring up assistants who can co-operate with him in a great work that brings credit both to himself, to themselves, and to the institute which they all represent.
Ralph Abercromby.
[Continued from Supplement, No. 610, page 9744.]
[Journal Of The Society Of Chemical Industry.]
When I visited Canada in 1877-78, the refining of petroleum was principally conducted in the city of London, Ontario. At the present time Petrolia, Ontario, is the chief seat of the industry, and it was accordingly to this city that we made our way. Here we were treated with the greatest kindness and hospitality by Mr. John D. Noble, vice-president of the Petrolia Crude Oil and Tanking Co., and his brother, Mr. R. D'Oyley Noble, and were enabled in the short time at our disposal to visit typical portions of the producing territory and some of the principal refineries.
The development of the Canadian petroleum industry may be said to date from 1857, when a well dug for water was found to yield a considerable quantity of petroleum; but long previously, indeed from the time of the earliest settlements in the county of Lamberton, in the western part of the province of Ontario, petroleum was known to exist in Canada. In 1862 productive flowing wells were drilled at Oil Springs, but these wells, which were comparatively shallow, quickly became exhausted, and the territory was deserted on the discovery in 1865 of oil at Petrolia, seven miles to the northward, and about 16 miles southwest of the outlet of Lake Huron. Recently the Oil Springs wells have been drilled deeper, and are now producing 10,000 to 12,000 barrels (of 42 American gallons) per month. Petroleum has also been found at Bothwell, 35 miles from Oil Springs, but this district has ceased to yield. Quite recently a new territory has been discovered at Euphemia, 17 miles from Bothwell, where, at the time of our visit, there were four wells producing collectively 70 barrels per day. This territory is by some regarded as part of the Bothwell field.
The present producing oil belt extends from Petrolia in a northwesterly direction, to the township of Sarnia, and in a southeasterly direction to Oil Springs, but in the latter direction there is a break of about four and a quarter miles, commencing at a point about two miles from Petrolia. At Oil Springs there appears to be a pool about two miles square. The extension of the belt then continues in the same direction, with another break of about nine miles, to the new oil field of Euphemia, the average width of the oil belt being about two miles. In all, about 15,000 wells are believed to have been drilled in the Canadian oil fields, and of these about 2,500 are now producing, the average yield being about three quarters of a barrel per well per day. The aggregate production is probably about 700,000 barrels per annum, the greater part of which is obtained in the Petrolia district, and the stocks were at the time of our visit stated to amount to from 400,000 to 450,000 barrels.
In the Canadian oil fields the drilling contractor usually employs his own derrick, engine, boiler, and tools, furnishes wood and water, cases the well, and fixes the pump; the well owner providing the casing and pump, and subsequently erecting the permanent derrick.
The wells in the Oil Springs field were formerly from 200 ft. to 300 ft. in depth, but the oil stratum then worked became waterlogged, and the wells are now sunk to a depth of about 375 ft., and are cased to a depth of about 275 ft. to shut off the water. The contract price for drilling a 4⅝ in. hole to a depth of about 375 ft. under the conditions mentioned is 150 dols. (£30), and the time occupied in drilling is usually about a week when the work is continued night and day. The wells in the Petrolia field have a depth of 480 ft., the contract price, including the cost of 100 ft. of wooden conductor, being 175 dols. (£35), and the time occupied in drilling being from six to twelve days. Pole tools are used in drilling, the poles being of white ash, 37 ft. in length. The derrick is about 48 ft. in height. An auger some 4 ft. in length, and about a foot in diameter, is used to bore through the earth to the bed rock, the auger being rotated by horse power.
The drilling tools commonly consist of a bit, 2½ ft. in length by 4⅝ in. in diameter, weighing about 60 lb.; a sinker bar, into which the bit is screwed, 30 ft. in length by 3 in. in diameter, weighing about 1,040 lb.; and the jars, inserted between the sinker bar and the poles about 6 ft. in length, and weighing 150 lb. The tools are suspended by a chain, which passes three times round the end of the walking beam and thence to the windlass, with ratchet wheel fixed on the walking beam, by means of which the tools are gradually lowered as the drilling proceeds. The cable is thus only employed in raising the tools from the well and lowering them into it.
The sand pump or bailer is frequently as much as 37 ft. in length, and is about 4 in. in diameter. The casing (4⅝ in diameter) costs about 45 cents (1s. 10½d.) per foot, and the 1¼ in. pump, with piping, costs from 65 dols. (£13) to 85 dols. (£17), according to the length of pipe required. An ordinary square frame derrick costs, with mud sill, from 22 dols. (£4 8s.) to 27 dols. (£5 8s.), and the walking beam about 8 dols. (£1 12s.) In many cases, however, a three-pole derrick, which can be erected at an expense of about 10 dols. (£2), is employed. A 100 barrel wooden tank costs, erected, 50 dols. (£10).
The wells are torpedoed on completion with from 8 to 10 quarts of nitroglycerine, at a cost of 4 dols. (16s.) per quart. The torpedoes employed in the Canadian oil field are much smaller than those used for a similar purpose in the United States, the tin shell being only 6 ft. in length by 3 in. in diameter. We were enabled to witness the operation of torpedoing a well, and the following particulars, based on notes taken at the time, may be of interest: The torpedo case, which was furnished with a tube or "anchor" at the lower end, 8 ft. in length, was placed in the mouth of the well and suspended so that its upper end was level with the surface of the ground. Eight quarts of nitroglycerine, which was in a tin can, was then poured into the torpedo case, and the torpedo was carefully lowered into the well, which contained at the time about 250 ft. of water, until the end of the anchor rested on the bottom of the well. A traveling primer or "go-devil squib" was then prepared as follows: A tin cone, 14 in. in length by 2 in. in diameter at the open end, was partially filled with sand to give it the necessary weight. A piece of double tape fuse, 2 ft. long, was inserted into a Nobel's treble detonator, and over the detonator and a portion of the fuse a perforated tin tube or sheath was passed. This tube was then inserted through a hole in a strip of tin fixed across the mouth of the conical cup into the sand, so that the detonator was embedded. The sand was then saturated with nitroglycerine, the fuse lighted, and the primer dropped into the well. In about 45 seconds there was a perceptible tremor of the ground, immediately followed by a slight sound of the explosion. After an interval of a second or two there was a gurgling noise, and a magnificent black fountain shot up twice as high as the derrick, upon which all the spectators ran for shelter from the impending shower of oil and water. The well not being a flowing one, the outrush was only of momentary duration, and within a few minutes the drillers were at work removing from the well, by means of the sand pump, the fragments of rock which had been detached by the explosion. On the table are specimens of this rock, which I obtained at the time.
The maximum yield per well is ten barrels per day, and the minimum yield for which it is considered profitable to pump is a quarter of a barrel per day. The yield being in some cases so small, it is usual to pump a number of wells through the agency of one engine, the various pumps being connected with the motor by means of wooden rods. In one instance I saw as many as eighty wells being thus pumped from one center. The motive power was a 70 h.p. engine, which communicated motion, similar to that of the balance wheel of a watch, to a large horizontal wheel. From this wheel six main rod lines radiated, the length of stroke of the main lines being 16 in., and the rate of movement 32 strokes per minute. Some of the wells being pumped from this center were from one-half to three-quarters of a mile distant, and altogether about eight miles of rods were employed in the pumping of the eighty wells.
The pipe line system in Canada has not been fully developed, and accordingly the well owner has to convey his oil by road to the nearest receiving station. Thus from the Euphemia oil field the oil has to be "teamed" 17 miles, to Bothwell. For the conveyance of the oil by road a long and slightly conical wooden tank or barrel, resting horizontally on a wagon, is employed. These vessels hold from eight to ten barrels of oil. The Petrolia Crude Oil and Tanking Company is the principal transporting and storing company. The storage charge is one cent (½d.) per barrel per month, and the delivery charge two cents per barrel. The petroleum produced in the Oil Springs field is stored separately from that obtained in the Petrolia field.
The storage takes place for the most part in large underground tanks excavated in the retentive clay. These remarkable tanks are often as much as 30 ft. in diameter by 60 ft. in depth, and hold from 5,000 to 8,000 barrels. In the construction of the tanks the alluvial soil, of which there is about 18 ft. or 20 ft. above the clay, is curbed with wood and thoroughly puddled with clay. On the completion of the excavation, the entire vertical surface is then lined with rings of pine wood, so that the upper part down to the solid clay is doubly lined. The bottom is not lined. The roof of the tank is of wood, covered with clay. The cost of such a tank is about 22 cents (11d.) per barrel, or 1,760 dols. (£363) for an 8,000 barrel tank, and the time occupied in making such a tank is about six weeks.
The crude petroleum from the Petrolia field usually has a specific gravity ranging from 0.859 to 0.877, while the specific gravity of the petroleum from the Oil Springs field ranges from 0.844 to 0.854.
The oil occurs in the corniferous limestone, and buildings constructed of this stone frequently exude petroleum in hot weather.
Canadian crude petroleum is of a black color, and possesses a very disagreeable odor, due to the presence of sulphur compounds. These characteristics are shown by the samples on the table, for some of which I am indebted to Mr. James Kerr, secretary of the Petrolia Oil Exchange.
The stills used in the process of refining the crude oil are horizontal two-flued cylinders, 30 ft. in length by 10 ft. in diameter, provided with six 2 in. vapor pipes. The charge is 260 barrels, and the following is an outline of the method of working. Assuming the still to be charged on Monday morning, heating is commenced about 7 A.M., and the naphtha begins to come over about 8 A.M. Of this product about six barrels is obtained in the case of Petrolia crude, or 7½ barrels in the case of Oil Springs crude. The distillation of the naphtha takes from 2 to 3 hours, say till 10:30 A.M. The heat is then increased, and the distillation of the kerosene commences about noon, and continues till about 10 P.M. Of the kerosene distillate about 80 barrels are obtained. The first portion of the kerosene distillate is usually collected separately, is steamed to drive off the more volatile hydrocarbons, and is then mixed with the remainder of the kerosene distillate. The product which then commences to distill is known as tailings. This is collected separately and is redistilled. The distillation of the tailings continues till about 5 A.M. on Wednesday, by which time about 80 barrels has been obtained. Steam is then passed into the still through a perforated pipe extending to the bottom, and about 21 barrels of "gas oil" is distilled over. The additional quantity of kerosene obtained on redistilling the tailings brings up the total yield of this product to about 42 per cent. of the crude oil. The gas oil is sold for the manufacture of illuminating gas. The residue is distilled for lubricating oils and paraffin.
The agitator in which the kerosene distillate is treated commonly takes a charge of 465 barrels. To this quantity of distillate two carboys of oil of vitriol is added, and the oil and acid are agitated together for 20 minutes. The tarry acid having been allowed to settle is drawn off, and seven carboys more of acid is added. Agitation having been effected for 30 or 40 minutes, the tarry acid is removed as before. Another similar treatment with seven carboys of acid follows, and occasionally a fourth addition of acid is made. The oil is next allowed to remain at rest for an hour, any acid which settles out being drawn off, and cold (or, in winter, slightly warmed) water is allowed to pass down through the oil in fine streams, this treatment being continued, without agitation of the oil, for half an hour, or until the dark color which the oil assumed on treatment with acid is removed. The water is then drawn off, 10 barrels of solution of caustic soda (sp. gr. 15° B.) is added, and agitation conducted for 15 minutes. The caustic soda solution having been drawn off, 30 barrels of a solution of litharge in caustic soda is added. This solution is made by dissolving caustic soda in water to a density of 18° B. and then adding the litharge. Agitation with this solution is continued for about six hours, or until the oil is thoroughly deodorized. About 100 lb. of sublimed sulphur is then added, and the agitation is continued for another two hours. The oil having been allowed to settle all night, the litharge solution is drawn off, and the oil run into a shallow tank or "bleacher," where it is exposed to the light to improve its color, and is, if necessary, steamed to drive off the lighter hydrocarbons and raise the flashing point to the legal minimum of 95° F. To raise the flashing point from 73° F. to 95° F. (Abel test) is stated to involve in practice a loss of 10 per cent., the burning quality of the oil being at the same time seriously impaired, and upon this ground the Ontario refiners in 1886 petitioned for a reduction of the test standard.
The average percentage yield of the various products is given in the following table:
| Naphtha. | 5 |
| Kerosene. | 42 |
| Gas oil. | 8 |
| Tar. | 25 |
| Coke. | 10 |
| Loss (including water). | 10 |
| 100 |
There are a dozen petroleum refineries in Canada, and the annual outturn of kerosene is about 200,000 barrels of 45 imperial gallons per annum. The total consumption of kerosene in Canada is about 300,000 barrels, one-third of which is manufactured in the United States. The United States oil is subject to a duty of 40 cents on the package and 7-1/5 cents per imperial gallon on the contents, besides which there is an inspection fee of 30 cents per package. Of lubricating oils the outturn is from 75,000 to 100,000 barrels per annum.
The quality of Canadian kerosene has been greatly improved of late years, but notwithstanding the elaborate process of refining, the oil, though thoroughly deodorized and of good color, contains sulphur, and of course evolves sulphur compounds in its combustion.
The rules of the Petrolia Oil Exchange provide that refined kerosene shall be of the odor "locally known as inoffensive," and shall "absolutely stand the test of oxide of lead in a strong solution of caustic soda without change of color."
The "burning percentage" in the case of "Extra Refined Oil," "Water White" in color, and of specific gravity not exceeding 0.800, is required to be not less than 70; in the case of "No. 1 Refined Oil," "Prime White" in color, not less than 60; and in the case of "No. 2 Refined Oil," "Standard White" in color, to be not less than 55.
The "burning percentage" is determined by the use of a lamp thus described: "The bowl of the lamp is cylindrical, 4 in. in diameter and 2¾ in. deep, with a neck placed thereon of such a height that the top of the wick tube is 3 in. above the bowl. A sun-hinge burner is used, taking a wick 7/8 in. wide and 1/8 in. thick, and a chimney about 8 in. long." The test is conducted as follows: "The lamp bowl is filled with the oil and weighed, then lighted and turned up full flame just below the smoking point, and burned without interference till 12 oz. of the oil is consumed. The quantity consumed during the first hour and the last hour is noted." The ratio of the two quantities is the measure of the burning quality, and the percentage that the latter quantity is of the former is the "burning percentage" referred to.
As this is the usual time of the year for planting, pruning, and removing forest trees and shrubs, it is a fit time for considering the influence which trees exert on the sanitary surroundings of dwelling places. The recent parliamentary report on forestry shows that trees are now of little commercial value in this country. And we may conclude, therefore, that they are chiefly grown for picturesque effect, and for the shelter from the sun and winds which they afford.
The relation of forests to rainfall has been studied by meteorologists, but little attention has been given by medical climatologists to the share which trees take in determining local variations of climate and the sanitary condition of dwellings, notwithstanding they play as important a part as differences of soil, of which so much is said and written nowadays. This remark does not apply to large towns, where trees grow with difficulty and are comparatively few in number, and where they afford a grateful relief to the eye, shade from the sun, and to a very slight extent temper the too dry atmosphere, but to suburban and country districts, where it is the custom to bury houses in masses of foliage—a condition of things which is deemed the chief attraction, and often a necessary accompaniment, of country life.
Trees of all kinds exercise a cooling and moistening influence on the atmosphere and soil in which they grow. The extent of these conditions depends on the number of trees and whether they stand alone, in belts, or in forests; on their size, whether tall trees with branchless stems or thickets of underwood: on their species, whether deciduous or evergreen; and on the season of the year. The cooling of the air and soil is due to the evaporation of water by the leaves, which is chiefly drawn from the subsoil—not the surface—by the roots, and to the exclusion of the sun's rays from the ground, trees themselves being little susceptible of receiving and radiating heat. The moisture of the atmosphere and ground about trees is due to the collection by the leaves and branches of a considerable portion of the rainfall, the condensation of aqueous vapor by the leaves, and the obstruction offered by the foliage to evaporation from the ground beneath the trees.
The experiments of M. Fautrat show that the leafage of leaf bearing trees intercepts one-third, and that of pine trees the half, of the rainfall, which is afterward returned to the atmosphere by evaporation. On the other hand, these same leaves and branches restrain the evaporation of the water which reaches the ground, and that evaporation is nearly four times less under a mass of foliage in a forest, and two and one-third times under a mass of pines, than in the open. Moreover, trees prevent the circulation of the air by lateral wind currents and produce stagnation. Hence, as Mr. E.J. Symons has truly observed, "a lovely spot embowered in trees and embraced by hills is usually characterized by a damp, misty, cold, and stagnant atmosphere," a condition of climate which is obviously unfavorable to good health and especially favorable to the development of consumption and rheumatism, our two most prevalent diseases.
Now, if we examine the surroundings of many of our suburban villas and country houses of the better sort, we shall find them embowered in trees, and subject to all the insanitary climatic conditions just mentioned. The custom almost everywhere prevails of blocking out of view other houses, roads, etc., by belts of trees, often planted on raised mounds of earth, and surrounded by high close walls or palings, from a foolish ambition of seeming to live "quite in the country."
This is a most unwise proceeding from a sanitary point of view, and should be protested against as strongly by medical men as defective drainage and bad water supply. Many houses stand under the very drip and shadow of trees, and "the grounds" of others are inclosed by dense belts of trees and shrubs, which convert them into veritable reservoirs of damp, stagnant air, often loaded with the effluvia of decaying leaves and other garden refuse, a condition of atmosphere very injurious to health, and answerable for much of the neuralgia of a malarious kind, of which we have heard so much lately. A very slight belt of trees suffices to obstruct the lateral circulation of the air, and if the sun be also excluded the natural upward currents are also prevented.
As far back as 1695 Lancisi recognized the influence of slight belts of trees in preventing the spread of malaria in Rome, and the cold, damp, stagnant air of spaces inclosed by trees is easily demonstrated by the wet and dry bulb thermometer, or even by the ordinary sensations of the body. A dry garden, on gravel, of three acres in extent in Surrey, surrounded by trees, is generally three or four degrees colder than the open common beyond the trees; and a large pond in a pine wood twenty miles from London afforded skating for ninety consecutive days in the winter of 1885-86, while during the greater part of the time the lakes in the London parks were free from ice.
The speculative builder has more sins to answer for than the faulty construction of houses. He generally begins his operations by cutting down all the fine old trees which occupy the ground, and which from their size and isolation are more beautiful than young ones and are little likely to be injurious to health, and ends them by raising mounds and sticking into them dense belts of quick-growing trees like poplars to hide as speedily as possible the desolation of bricks and mortar he has created. It is this senseless outdoor work of the builder and his nurseryman which stands most in need of revision from time to time in suburban residences, but which rarely receives it from a silly notion, amounting to tree worship, which prohibits the cutting down of trees, no matter how injudicious may have been the planting of them in the first instance from a sanitary or picturesque point of view.
The following hints for planting and removing trees may be useful to those persons who have given little attention to the subject. A tree should not stand so near a house that, if it were to fall, it would fall on the house; or, in other words, the root should be as far from the house as the height of the tree. Belts of trees may be planted on the north and east aspects of houses, but on the east side the trees should not be so near, nor so high, as to keep the morning sun from the bedroom windows in the shorter days of the year. On the south and west aspects of houses isolated trees only should be permitted, so that there may be free access of the sunshine and the west winds to the house and grounds.
High walls and palings on these aspects are also objectionable, and should be replaced by fences, or better still open palings, especially about houses which are occupied during the fall of the leaf, and in the winter. Trees for planting near houses should be chosen in the following order: Conifers, birch, acacia, beech, oak, elm, lime, and poplar. Pine trees are the best of all trees for this purpose, as they collect the greatest amount of rainfall and permit the freest evaporation from the ground, while their branchless stems offer the least resistance to the lateral circulation of the air.
Acacias, oaks, and birches are late to burst into leaf, and therefore allow the ground to be warmed by the sun's rays in the early spring. The elm, lime, and chestnut are the least desirable kinds of trees to plant near houses, although they are the most common. They come into leaf early and cast their leaves early, so that they exclude the spring sun and do not afford much shade in the hot autumn months, when it is most required. The lime and the elm are, however, beautiful trees, and will doubtless on this account often be tolerated nearer houses than is desirable from a purely sanitary point of view.
Trees are often useful guides to the selection of residences. Numerous trees with rich foliage and a rank undergrowth of ferns or moss indicate a damp, stagnant atmosphere; while abundance of flowers and fruit imply a dry, sunny climate. Children will be healthiest where most flowers grow, and old people will live longest where our common fruits ripen best, as these conditions of vegetation indicate a climate which is least favorable to bronchitis and rheumatism. Pines and their companions, the birches, indicate a dry, rocky, sandy, or gravel soil; beeches, a dryish, chalky, or gravel soil; elms and limes, a rich and somewhat damp soil; oaks and ashes, a heavy clay soil; and poplars and willows, a low, damp, or marshy soil. Many of these are found growing together, and it is only when one species predominates in number and vigor that it is truly characteristic of the soil and that portion of the atmosphere in connection with it.
Curzon Street, Mayfair, W.—Lancet.
M. Amagat has succeeded in solidifying various liquids, by compressing them in cylinders of bronze and steel. He has also photographed the crystals after crystallization, by means of a ray of electric light traversing the interior of the vessel by glass cones serving as panes. The stages of crystallization can be observed in this way with chloride of carbon, and it is seen that the process varies with the rapidity with which the pressure is produced. If rapidly, a sudden circlet of crystals gathers round the edge of the luminous field, and grows to the center. The pressure being continued, the field becomes obscure, then transparent. As the pressure is diminished the reverse takes place, and the liquid state is reproduced. M. Amagat finds that chloride of carbon solidifies at 19.5° Cent., under a pressure of 210 atmospheres. At 22° Cent., benzine crystallizes with a pressure of about 900 atmospheres.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of THE SUPPLEMENT, from the commencement, January 1, 1876, can be had. Price, 10 cents each.
All the back volumes of THE SUPPLEMENT can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50 stitched in paper, or $3.50 bound in stiff covers.
COMBINED RATES.—One copy of SCIENTIFIC AMERICAN and one copy of SCIENTIFIC AMERICAN SUPPLEMENT, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
361 Broadway, New York, N. Y.
In connection with the Scientific American, Messrs. MUNN & Co. are solicitors of American and Foreign Patents, have had 42 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to MUNN & Co.
We also send free our Hand Book about the Patent Laws, Patents, Caveats, Trade Marks, their costs, and how procured. Address
Munn & Co.,
361 Broadway, New York.
Branch Office, 622 and 624 F St., Washington, D.C.
This is a Special Edition of the Scientific American, issued monthly—on the first day of the month. Each number contains about forty large quarto pages, equal to about two hundred ordinary book pages, forming, practically, a large and splendid Magazine of Architecture, richly adorned with elegant plates in colors and with fine engravings, illustrating the most interesting examples of modern Architectural Construction and allied subjects.
A special feature is the presentation in each number of a variety of the latest and best plans for private residences, city and country, including those of very moderate cost as well as the more expensive. Drawings in perspective and in color are given, together with full Plans, Specifications, Costs, Bills of Estimate, and Sheets of Details.
No other building paper contains so many plans, details, and specifications regularly presented as the Scientific American. Hundreds of dwellings have already been erected on the various plans we have issued during the past year, and many others are in process of construction.
Architects, Builders, and Owners will find this work valuable in furnishing fresh and useful suggestions. All who contemplate building or improving homes, or erecting structures of any kind, have before them in this work an almost endless series of the latest and best examples from which to make selections, thus saving time and money.
Many other subjects, including Sewerage, Piping, Lighting, Warming, Ventilating, Decorating, Laying out of Grounds, etc., are illustrated. An extensive Compendium of Manufacturers' Announcements is also given, in which the most reliable and approved Building Materials, Goods, Machines, Tools, and Appliances are described and illustrated, with addresses of the makers, etc.
The fullness, richness, cheapness, and convenience of this work have won for it the Largest Circulation of any Architectural publication in the world.
MUNN & CO., Publishers,
361 Broadway, New York.
A Catalogue of valuable books on Architecture, Building, Carpentry, Masonry, Heating, Warming, Lighting, Ventilation, and all branches of industry pertaining to the art of Building, is supplied free of charge, sent to any address.
In connection with the publication of the Building Edition of the Scientific American, Messrs. Munn & Co. furnish plans and specifications for buildings of every kind, including Churches, Schools, Stores, Dwellings, Carriage Houses, Barns, etc.
In this work they are assisted by able and experienced architects. Full plans, details, and specifications for the various buildings illustrated in this paper can be supplied.
Those who contemplate building, or who wish to alter, improve, extend, or add to existing buildings, whether wings, porches, bay windows, or attic rooms, are invited to communicate with the undersigned. Our work extends to all parts of the country. Estimates, plans, and drawings promptly prepared. Terms moderate. Address
Munn & Co., 361 Broadway, New York.
End of the Project Gutenberg EBook of Scientific American Supplement, No.
611, September 17, 1887, by Various
*** END OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN ***
***** This file should be named 16948-h.htm or 16948-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/1/6/9/4/16948/
Produced by Juliet Sutherland and the Online Distributed
Proofreading Team at www.pgdp.net
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
http://gutenberg.net/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.net
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.net),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH F3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need, is critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at http://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
http://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
business@pglaf.org. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at http://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit http://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including including checks, online payments and credit card
donations. To donate, please visit: http://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart is the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
http://www.gutenberg.net
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.